Graphene represents a new generation of materials which exhibit unique physicochemical properties such as high electron mobility, tunable optics, a large surface to volume ratio, and robust mechanical strength. These properties make graphene an ideal candidate for various optoelectronic, photonics, and sensing applications. . In recent years, numerous efforts have been focused on azobenzene polymers (AZO-polymers) as photochromic molecular switches and thermal sensors because of their light induced conformations and surface-relief structures. However, these polymers often exhibit drawbacks like low photon storage lifetime, energy density and aggregation. These issues can be alleviated by incorporating graphene derivatives (GDs) into AZO-polymers to form orderly arranged molecules like GD-AZO composites.
1. Introduction
Nanotechnology stands at the forefront of humanity’s most fervent desires of progression in virtually all aspects: health, engineering, space exploration, and tying these all together—nanomaterials. Graphene derivatives (GDs) have led to a new generation of multifunctional nanomaterials with unique properties such as a large surface area, a tunable microstructure, an adjustable bandgap, high electronic conductivity, robust mechanical strength, and thermal storage
[1][2][1,2]. Properties of GDs can be modulated by functionalization, i.e., coupling them with a variety of compounds that lead to new building blocks with advanced properties
[3][4][3,4]. Functionalization provides avenues for interfacial interactions enabling better dispersion or solubility samples, leading to improved processability
[5][6][5,6]. Thus, many efforts are directed at amalgamations of different materials with GDs to form more sophisticated and effective structures with greater potential for various applications
[7][8][7,8]. Among the variegated nanomaterials composited with GDs, photochromic compounds could be a perfect choice for GDs’ functionalization needed for optoelectronic and thermal applications
[9][10][11][12][9,10,11,12].
Photochromic materials can undergo reversible photo-triggered isomerization between (at least) two (meta)stable states that exhibit distinct spectroscopic and physical properties
[13]. Various types of photochromic materials are currently considered as viable candidates for reversible information storage and optical switching applications
[14][15][14,15]. Among them, the most extensively studied photochromic compounds are azobenzene polymers (AZO-polymers). The light-induced conformations of azobenzene chromophores can result in large and stable in-plane anisotropy, nonlinear optical responses, and inscription of surface relief structures onto a material system
[16][17][18][19][16,17,18,19]. The photoisomerization of azobenzene moieties in interactive molecules or groups results in the distinctive switching of chemical, optical, steric, thermal, and electrical properties of functional composites, enabling them to be beneficial for various photonic and electronic applications
[20][21][20,21].
Recently, GDs functionalized in AZO-polymer complexes have been explored for producing a moiety capable of photo-modulation and solar thermal storage
[22][23][24][25][22,23,24,25]. Two-dimensional structures of GDs are an excellent platform for assembling close packed order AZO molecules. This allows for high density grafting, high intermolecular interactions, and steric hindrance which can be used to control steric configurations and functional groups in a composite system
[26][27][26,27]. Graphene derivative/azobenzene (GD-AZO) interactions lower the energy of the trans-isomer and stabilize the cis-isomer, leading to remarkable increases in both ΔH and t
1/2 of the molecules
[12]. The introduction of GDs in AZO-polymers also prevents the undesired aggregation effect which is detrimental for their optical response. In addition, GD-AZO composites exhibit extraordinary optical and electronic properties, including efficient charge transfer, enhanced quantum yield, changed electrical field, remarkable photo-stability and ultrafast kinetics
[28][29][28,29]. As such, it is crucial to review the intellectual progression rendered in this field thus far.
2. Properties of GDs, AZO-Polymers, and GD-AZO Composites
2.1. Properties of GDs
Novoselov et al. separated and characterized an atom-thick
sp2-hybridized carbon nanosheet and defined it as “isolated graphene” in 2004
[30][39]. Since its discovery, this nanomaterial has propelled enormous scientific and technological interest due to its unique properties such as a large specific surface area, easy functionalization, controlled photoluminescence, high mechanical strength, chemical stability, and high electronic conductivity
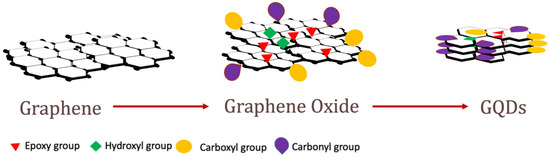
[1]. GO and GQDs are subsets of the graphene family which are usually derived from graphene by exfoliation reactions. The structures of graphene, GO nanosheets, and GQDs are shown in
Figure 1. GDs (e.g., GO, reduced graphene oxide, GQDs) have proven to be effective coupling materials for many polymer nanocomposites because of their ideal material properties and dispersibility in polymer matrices
[31][40]. The tight packing of
sp2 carbon, their electronic conjugation, and π–π transitions demonstrate their use in various sensor and optoelectronic devices
[32][33][41,42]. This review intends to highlight the properties of GDs as coupling agents for AZO-polymers.
Figure 1. Structure of Graphene, Graphene Oxide and GQDs.
2.2. Properties of AZO-Polymers
Azobenzene is an aromatic molecule in which two phenyl rings are bridged by an AZO linkage (–N=N–). The extended conjugation exhibited by these molecules gives rise to their strong absorption in UV and VIS light ranges
[34][62]. Owing to their colorant properties, AZO-polymers have been used as dyes and pigments for over 170 years
[35][63]. However, photochromic properties and reversible trans ⇌ cis isomerizations of these materials were elucidated in the first half of the 1900s
[36][37][64,65]. Trans ⇌ cis photoisomerization is the basic molecular level process in which a thermally stable trans-state is converted to a metastable cis state upon the absorption of a photon
[38][66]. This photoisomerization leads to the dramatic changes in the properties of azobenzene including their optical absorption, redox behavior, optical linearity, and surface relief gratings (SRGs).
2.3. Properties of GD-AZO Composites
The functionalization of GDs such as GO and GQDs with AZO moieties is essential to modulate properties of composite systems which can be further tuned for use in various applications. For example, functionalization with GDs improves the stability and dispersion of AZO moieties in water which is very important for their biological applications
[39][95]. In addition, GD-AZO composites show extraordinary optical and electronic properties, such as efficient charge transfer, enhanced quantum yields, changed electrical fields, remarkable photostability, and ultrafast kinetics.
3. Synthesis of GD-AZO Composites
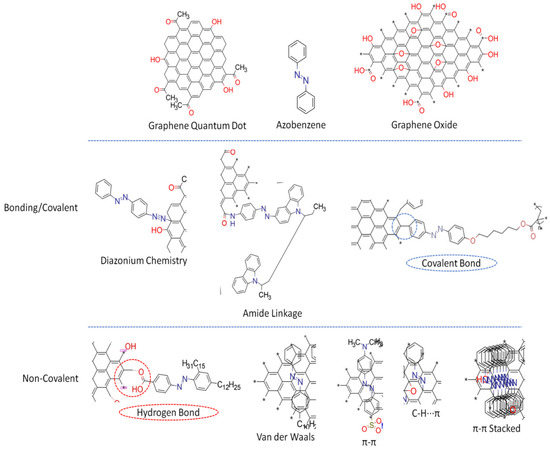
The efficient integration of photo-responsive azobenzene into GDs is an important factor in manipulating their thermal and electrical properties, potentially making them easier to work with in a wider range of applications. The fundamental factor involved in the synthesis of a GD-AZO composite is the interaction between surface functional groups present on the GDs, and small functional groups present on the AZO-polymer chains. For optimum interaction, GDs and the AZO-polymer matrix must be highly soluble in a common solvent. This enables the composite to be synthesized in a way that charge transfer can be maximized. Maximized charge transfer at the interface is important for the fabrication of high-performance conducting nanocomposites. The interaction chemistry between GDs and AZO-polymers includes covalent and non-covalent functionalization. In both cases, it is important to have a well-defined connection between GDs and azobenzenes; if the interaction is weak, the composite formation turns out to be inefficient for most desirable applications
[25]. Bujak et al. explain how covalently functionalized systems possess an advantage over non-covalently functionalized systems by leading to improved thermal stability of the azobenzene, without conferring the disadvantages of “guest–host” AZO systems
[40][100]. In this regard, covalent functionalization is more advantageous than non-covalent. However, a drawback of covalent functionalization on a graphene surface is the deterioration of the properties related to the transport of electrons or phonons due to the conversion of
sp2 carbon into
sp3. The functionalization and various types of linkages between the GDs and azobenzenes are summarized in
Figure 2.
Figure 2. Synthesis strategies for GD-AZO composites
[41][87].
3.1. Covalent Linkages
Covalent functionalization involves strong molecular bonding between GDs and azobenzenes, which inevitably results in partial damage of π-conjugated structures. However, strong chemical bonds at an interface facilitate precise control and modulation of electronic properties of the composite material, contributing to efficient charge or energy transfer and quantum effects
[42][43][101,102]. Many GD-AZO composites synthesized by covalent functionalization develop non-covalent interactions after composite formation, significantly influencing the properties of the composites.
GO and GQDs have carboxylic and other functional groups on their surfaces that can be readily used for covalent modifications
[44][103]. Condensation reactions, such as diazotization and amidation reactions, are the preferred approaches. The carboxylic groups on the surfaces of GDs are activated by generating the corresponding acid chloride which reacts with suitable azobenzene moieties carrying OH or NH
2 reactive groups.
3.2. Non-Covalent Linkage
Non-covalent attachment enables GD-AZO composites to preserve high-quality π-conjugated structures
[45][109]. This induces macroscopic effects such as photoisomerization, photocontrol molecular alignment, and photoinduced surface patterning in the composite molecule
[46][110]. Non-covalent functionalization usually occurs by physical adsorption of modified azobenzene molecules on GDs using π–π stacking and electrostatic interactions.
4. Characterization Techniques
The functionalization of GD-AZO composites through covalent and non-covalent stacking or polymerization has been explored by various characterization techniques. These techniques mostly rely on material property changes induced by covalent and non-covalent functionalization. Some of these techniques are discussed below.
4.1. Fourier Transform Infrared Spectroscopy
Fourier-transform infrared (FTIR) spectroscopy is the most common technique for characterizing chemical functional groups present in the polymer nanocomposites. The shift in the IR wavenumbers after functionalization can be qualitatively correlated with the covalent or non-covalent interactions between GDs and AZO moieties
[47][117]. The major bands that appear in the composites are due to the presence of aromatic rings, AZO chromophores (–N=N–), C–N stretching, along with other bands. Some of the major functional groups present in the FTIR spectra of the composites and their corresponding wavenumbers are listed in
Table 1 [48][118].
Table 1. Functional groups present in GD-AZO composites.
|
| Wavenumbers |
| (cm −1) |
|
| Assigned Functional Groups |
|
|
| 3434 |
|
| O=H Stretching |
|
|
| 1728 |
|
| C=O Stretching |
|
|
| 1640 |
|
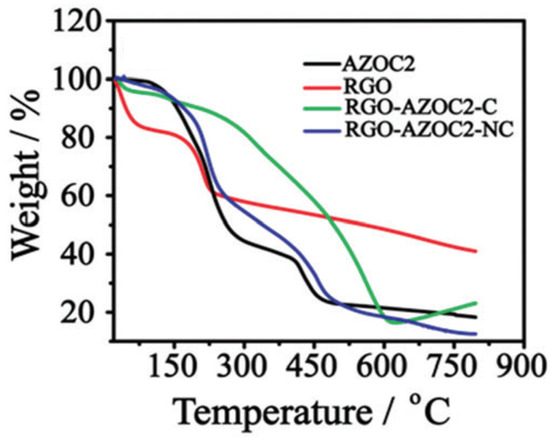
As can be seen from
Figure 5, RGO shows main mass loss from 140 to 250 °C due to the loss of carboxylic acid groups. AZOC2 shows a two-step weight loss because of the larger rupture of the azobenzene backbone, as well as the elimination of carboxylic acid groups. Upon assembly formation with AZOC2, there is a comparatively lower rate of mass loss which clearly points to the interaction of AZOC2 with RGO. The covalent and non-covalent assemblies formed between RGO and AZOC2 are relatively stable as evident from their lower decomposition rates. The thermal stability of RGO-AZOC2-NC is found to be slightly better than the RGO-AZO-C moieties.
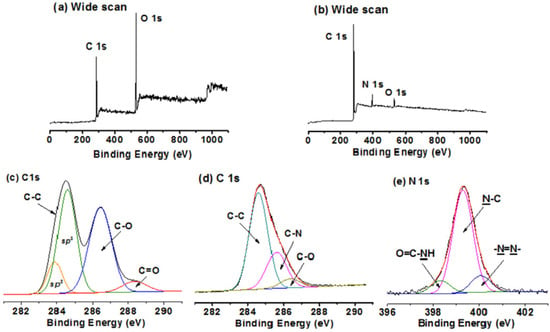
4.5. X-ray Photoelectron Spectroscopy Analysis
The density of AZO moieties grafted onto GO/RGO was obtained by X-ray photoelectron spectroscopy (XPS) analysis. The density was estimated from the content variation of C and N in the wide scan survey XPS of RGO, AZO moieties, and RGO-AZO hybrid materials. Zhang et al. synthesized an unconjugated poly(N-vinylcarbazole)(PVK) pendant azobenzene chromophores (PVK-AZO)
[53][107]. These chromophores were successfully covalently grafted onto the surface of GO via the amide linkage to produce a new polymer covalently grafted GO-functional material, PVK-AZO-GO. The XPS spectra of these polymers are shown in
Figure 6.
Figure 6. Wide scan and C1s core-level XPS spectra of (
a,
c) GO, and (
b,
d) PVK-AZO-GO; N1s core-level XPS spectra of € PVK-AZO-GO (Reprinted with permission from
[53][107]; Copyright 2018 Elsevier).
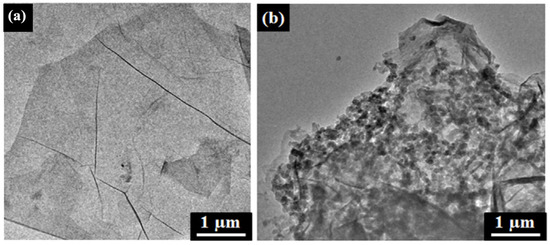
The interaction between RGO and AZO molecules can be observed by transmission electron microscopy (TEM) analysis. The TEM images of the GO and GO-AZO composites synthesized by Zhang et al. are shown in
Figure 7a,b, respectively
[53][107].
Figure 7a shows a transparent layered and wrinkled silk-like structure of GO. In contrast to GO, the morphology of GO-AZO (
Figure 7b) looks like a plane-stacked rigid structure in which the surface of GO was surrounded by the pellet-like AZO moieties. After the grafting of the AZO molecules, the roughness of the GO nanosheets also increased because they were covered by many organic AZO addends.
Figure 7. TEM images of (
a) GO and (
b) PVK-AZO-GO (Reprinted with permission from
[53][107]; Copyright 2018 Elsevier).
5. Applications
Photo-induced changes in microstructures, electronic properties, optical responses, and steric effects of azobenzene moieties can be utilized to fabricate a variety of photo-energy conversion or storage devices. The functionalization of AZO moieties with GDs can reflect, extend, and amplify the optically modulated conductance, electrostatic response, absorption, and catalytic properties of the individual constituents of the composite
[54][121]. The favorable properties of GD-AZO composites include: (1) charge transfer in nanosecond range at the interface, (2) high quantum yields, (3) energy storage potential in chemical bonds, (4) controlled electrostatic environment around carbon π-conjugated structures, (5) electrochemical catalytic activity, and (6) ultrafast isomerization within a few picoseconds (10
−12 s)
[25]. These properties of GD-AZO composites are vital for the various applications listed in
Table 2. Investigations related to these GD-AZO applications are analyzed below.
Table 2. Applications of GD-AZO composites.
|
| Graphene |
| Derivative |
|
| Azobenzene |
| Derivative |
|
| Linkage Method |
| (Type of Linkage) |
|
| Application |
|
| Reference |
|
|
| GO and RGO |
|
| Azobenzene |
|
| Covalent |
| Diazotization method |
|
| Solar Thermal storage |
|
[55]
|
[104]
|
|
| RGO |
|
| Azobenzene |
|
| Covalent |
| Diazotization method |
|
| Solar Thermal storage |
|
[56]
|
[105]
|
|
| C=C Stretching |
|
|
| 1386 |
|
| C–O Stretching |
|
|
| 1060 |
|
| C–O–C Stretching |
|
|
| 1581 |
|
| N=N Stretching |
|
|
| 1297 |
|
| C–N stretching |
|
|
| 3060 |
|
| C–H stretching |
|
4.2. X-ray Diffraction Analysis
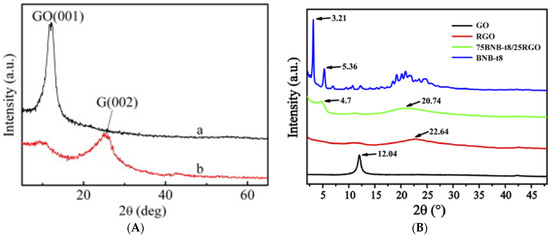
The effect of covalent and non-covalent AZO functionalization on the interlayer distance and the crystallization of GO and RGO can be analyzed by X-ray diffraction (XRD) spectra. The XRD patterns of the GO and GO-AZO composites synthesized by Zhang et al. through covalent (amide) linkages are shown in
Figure 3A
[49][97]. XRD patterns of BnB-t8(AZO-derivative)-RGO nanocomposites synthesized through non-covalent linkage (π–π stacking) are shown in
Figure 3B
[50][111].
Figure 3. XRD patterns of GD-AZO hybrids via (
A) Covalent (a) GO, (b) GO-AZO. (Reprinted from
[49][97]; Copyright 2010 Elsevier) Additionally, (
B) Non-covalent linkage
[50][111].
The XRD patterns of GO in
Figure 3A,B show sharp peaks centered at 2
θ = 12.1 which correspond to the (001) interplanar spacing of 0.73 nm. In the case of GO-AZO and BNB-t8-AZO hybrids, the (001) peak becomes broad and inconspicuous, shifting to a larger d-spacing. It has been proposed that the insertion of the AZO moieties through covalent and noncovalent linkages disturbed the electrostatic interactions between the GO/RGO sheets. This results in the exfoliation of the GO/RGO layers, forming a graphite-like structure. Hence, both covalent and non-covalent functionalization show similar effects on the XRD patterns of the GD-AZO composites.
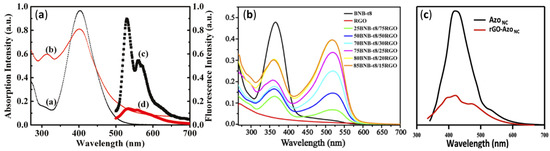
4.3. UV-Visible and Photoluminescence Spectroscopy
Optical properties of GD-AZO composites can be monitored using UV-Visible and photoluminescence (PL) spectroscopy. The UV-Visible and fluorescence spectra of AZO and GO-AZO composites synthesized by Zhang et al. using covalent linkages are shown in
Figure 4a
[51][119]. UV-Visible absorption and the corresponding PL spectra of non-covalently linked BnB-t8-RGO hybrids are given in
Figure 4b,c, respectively
[50][111].
Figure 4. (
a) UV-Visible spectra (a) GO, (b) GO-AZO and PL spectra of (c) GO and (d) GO-AZO samples by covalent linkage. (Reprinted from
[51][119]; Copyright 2010, American Chemical Society) (
b) UV-Visible absorption spectra of BNB-t8 (1 × 10
−3 M), RGO (2 mg/mL) and BNB-t8/RGO (2 mg of BNB-t8/RGO with various wt % of BNB-t8 sonicated in 1 mL of solvent for 5 min) in DMF and (
c) PL spectra of RGO and BnB-t8-RGO composites by non-covalent linkages
[50][111].
4.4. Thermogravimetric Analysis
The thermal stability of GD-AZO composites is investigated by the thermogravimetric analysis (TGA). Kizhisseri et al. synthesized RGO-AZO nanocomposites by covalent (RGO-AZOC2-C) and noncovalent linkages (RGO-AZOC2-NC)
[52][116]. The thermograms of the AZOC2 moiety, RGO, RGO-AZOC2-C and RGO-AZOC2-NC are shown in
Figure 5.
Figure 5. TGA curves of AZO moiety, RGO, RGO-AZOC2-C and RGO-AZOC2-NC hybrids. (Reprinted from
[52][116]; published by The Royal Society of Chemistry).
|
|
GO |
|
| Azobenzene |
|
| Covalent |
| Diazotization method |
|
| Potential |
| Supercapacitor |
| electrodes |
|
[57]
|
[106]
|
|
| GO |
|
| Azobenzene |
|
| Covalent |
| Amide linkage |
|
| Photoswitches |
|
[51]
|
[119]
|
|
| GO |
|
| Poly (N-vinylcarbazole) |
|
| Covalent |
| Amide linkage |
|
| Resistive random-access memory |
|
[53]
|
[107]
|
|
| GO |
|
| Azobenzene |
|
| Covalent |
| Amide linkage |
|
| Photoswitches |
|
[49]
|
[97]
|
|
| GO |
|
| Amino functionalized Azobenzene |
|
| Covalent |
| Polyimide method |
|
| Photoswitches |
|
[58]
|
[108]
|
|
| RGO |
|
| Polyazo (Bismark-Brown -Y) |
|
| Noncovalent |
| π–π stacking |
|
| Chemiresistor for dissolved O2 |
|
[59]
|
[112]
|
|
| RGO |
|
| Polyazo (Bismark-Brown -Y) |
|
| Noncovalent |
| π–π stacking |
|
| Chemiresistor sensor for mitochondrial |
| Oxygen consumption |
|
[60]
|
[122]
|
|
| RGO and GO |
|
| Azobenzene nanocluster |
|
| Noncovalent |
| π–π stacking |
| and direct immobilization |
|
| p-type diode |
| n-type diode |
|
[61]
|
[113]
|
|
| R-GO |
|
| Azobenzene BNB-t8 |
|
| Noncovalent |
| π–π stacking |
|
| Nonlinear optical material |
|
[50]
|
[111]
|
|
| RGO |
|
| Azobenzene from cardanol |
|
| Covalent |
| And Noncovalent |
|
| Photoswitches |
|
[52]
|
[116]
|
|
| RGO |
|
| Bis-azobenzene |
|
| Covalent bonding |
|
| Solar thermal storage |
|
[62]
|
[98]
|
|
| Au-doped |
| RGO |
|
| Gemini Azo |
|
| Noncovalent |
| Photochromic stabilizers |
|
| Photoswitches |
|
[63]
|
[115]
|
|
| GQDs |
|
| Azobenzene derived from cardanol |
|
| Noncovalent |
| Hydrogen bonding |
|
| Fluorescent Probe and |
| IMPLICATION logic gate |
|
[64]
|
[96]
|
|
| GO |
|
| Cationic surfactant azo |
|
| Noncovalent |
| Photochromic stabilizers |
|
| Photoswitches |
|
[65]
|
[114]
|