
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Seung Kew Yoon | + 2175 word(s) | 2175 | 2021-09-12 07:27:35 | | | |
| 2 | Conner Chen | Meta information modification | 2175 | 2021-10-08 11:03:01 | | |
Video Upload Options
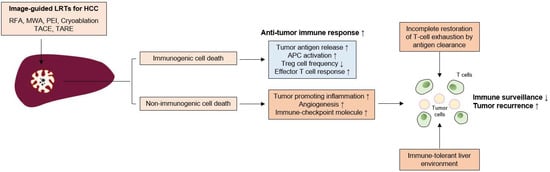
Hepatocellular carcinoma (HCC) is a common cause of cancer-related deaths worldwide. Unlike other types of cancer, HCC can be treated with locoregional treatments (LRTs) such as radiofrequency ablation (RFA) or transarterial chemoembolization (TACE). However, recurrences following LRTs are common, and strategies to improve long-term outcomes need to be developed. The exhaustion of anti-tumor immunity in HCC has been well established in many reports and the immunomodulatory effects of LRTs (enhancement of tumor antigen-specific T cell responses after RFA, reduction of effector regulatory T cells after TACE) have also been reported in several previous studies. However, a comprehensive review of previous studies and the possible roles of immunotherapy following LRTs in HCC are not known.
1. Effects of Cell Death in Anti-Tumor Immune Responses Induced by LRTs
2. Immunological Changes Following LRTs in HCC
2.1. Local Ablative Therapies
2.2. Transarterial Therapies
2.2.1. TACE
2.2.2. Transarterial Radioembolization (TARE)
References
- Tonnus, W.; Meyer, C.; Paliege, A.; Belavgeni, A.; Von Mässenhausen, A.; Bornstein, S.R.; Hugo, C.; Becker, J.U.; Linkermann, A. The pathological features of regulated necrosis. J. Pathol. 2019, 247, 697–707.
- Asadzadeh, Z.; Safarzadeh, E.; Safaei, S.; Baradaran, A.; Mohammadi, A.; Hajiasgharzadeh, K.; Derakhshani, A.; Argentiero, A.; Silvestris, N.; Baradaran, B. Current Approaches for Combination Therapy of Cancer: The Role of Immunogenic Cell Death. Cancers 2020, 12, 1047.
- Dudek-Peric, A.M.; Ferreira, G.B.; Muchowicz, A.; Wouters, J.; Prada, N.; Martin, S.; Kiviluoto, S.; Winiarska, M.; Boon, L.; Mathieu, C.; et al. Antitumor immunity triggered by melphalan is potentiated by melanoma cell surface-associated calreticulin. Cancer Res. 2015, 75, 1603–1614.
- Kepp, O.; Menger, L.; Vacchelli, E.; Adjemian, S.; Martins, I.; Ma, Y.; Sukkurwala, A.Q.; Michaud, M.; Galluzzi, L.; Zitvogel, L.; et al. Anticancer activity of cardiac glycosides: At the frontier between cell-autonomous and immunological effects. Oncoimmunology 2012, 1, 1640–1642.
- Greten, T.F.; Mauda-Havakuk, M.; Heinrich, B.; Korangy, F.; Wood, B. Combined locoregional-immunotherapy for liver cancer. J. Hepatol. 2019, 70, 999–1007.
- Den Brok, M.H.M.G.M.; Sutmuller, R.P.M.; Nierkens, S.; Bennink, E.J.; Frielink, C.; Toonen, L.W.J.; Boerman, O.C.; Figdor, C.; Ruers, T.J.M.; Adema, G.J. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br. J. Cancer 2006, 95, 896–905.
- Dromi, S.A.; Walsh, M.P.; Herby, S.; Traughber, B.; Xie, J.; Sharma, K.V.; Sekhar, K.P.; Luk, A.; Liewehr, D.J.; Dreher, M.R.; et al. Radiofrequency Ablation Induces Antigen-presenting Cell Infiltration and Amplification of Weak Tumor-induced Immunity. Radiology 2009, 251, 58–66.
- Scheffer, S.R.; Nave, H.; Korangy, F.; Schlote, K.; Pabst, R.; Jaffee, E.M.; Manns, M.P.; Greten, T.F. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int. J. Cancer 2003, 103, 205–211.
- Gamrekelashvili, J.; Kapanadze, T.; Han, M.; Wissing, J.; Ma, C.; Jaensch, L.; Manns, M.P.; Armstrong, T.; Jaffee, E.; White, A.O.; et al. Peptidases released by necrotic cells control CD8+ T cell cross-priming. J. Clin. Investig. 2013, 123, 4755–4768.
- Rai, R.; Richardson, C.; Flecknell, P.; Robertson, H.; Burt, A.; Manas, D. Study of Apoptosis and Heat Shock Protein (HSP) Expression in Hepatocytes Following Radiofrequency Ablation (RFA). J. Surg. Res. 2005, 129, 147–151.
- Vanagas, T.; Gulbinas, A.; Sadauskiene, I.; Dambrauskas, Z.; Pundzius, J.; Barauskas, G. Apoptosis is activated in an early period after radiofrequency ablation of liver tissue. Hepatogastroenterology 2009, 56, 1095–1099.
- Lu, W.; Li, Y.-H.; He, X.-F.; Zhao, J.-B.; Chen, Y.; Mei, Q.-L. Necrosis and Apoptosis in Hepatocellular Carcinoma Following Low-Dose Versus High-Dose Preoperative Chemoembolization. Cardiovasc. Interv. Radiol. 2008, 31, 1133–1140.
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714.
- Ding, Z.-C.; Lu, X.; Yu, M.; Lemos, H.D.P.; Huang, L.; Chandler, P.; Liu, K.; Walters, M.; Krasinski, A.; Mack, M.; et al. Immunosuppressive Myeloid Cells Induced by Chemotherapy Attenuate Antitumor CD4+ T-Cell Responses through the PD-1–PD-L1 Axis. Cancer Res. 2014, 74, 3441–3453.
- Schietinger, A.; Philip, M.; Krisnawan, V.E.; Chiu, E.Y.; Delrow, J.J.; Basom, R.S.; Lauer, P.; Brockstedt, D.G.; Knoblaugh, S.E.; Hammerling, G.J.; et al. Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity 2016, 45, 389–401.
- Ma, J.; Zheng, B.; Goswami, S.; Meng, L.; Zhang, D.; Cao, C.; Li, T.; Zhu, F.; Ma, L.; Zhang, Z.; et al. PD1(Hi) CD8(+) T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 331.
- Han, J.W.; Sung, P.S.; Kim, K.H.; Hong, S.-H.; Shin, E.-C.; Song, M.J.; Park, S.-H. Dynamic Changes in Ex Vivo T-Cell Function After Viral Clearance in Chronic HCV Infection. J. Infect. Dis. 2019, 220, 1290–1301.
- Li, G.; Staveley-O’Carroll, K.F.; Kimchi, E.T. Potential of Radiofrequency Ablation in Combination with Immunotherapy in the Treatment of Hepatocellular Carcinoma. J. Clin. Trials 2016, 6, 1–5.
- Jansen, M.C.; van Hillegersberg, R.; Schoots, I.G.; Levi, M.; Beek, J.F.; Crezee, H.; van Gulik, T.M. Cryoablation induces greater inflammatory and coagulative responses than radiofrequency ablation or laser induced thermotherapy in a rat liver model. Surgery 2010, 147, 686–695.
- Mizukoshi, E.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Arihara, F.; Kagaya, T.; Yamashita, T.; Fushimi, K.; Kaneko, S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013, 57, 1448–1457.
- Zhang, H.; Hou, X.; Cai, H.; Zhuang, X. Effects of microwave ablation on T-cell subsets and cytokines of patients with hepatocellular carcinoma. Minim. Invasive Ther. Allied Technol. 2017, 26, 207–211.
- De Bethlenfalva-Hora, C.E.; Piguet, A.C.; Schmitt, J.; Mertens, J.C.; Dufour, J.-F.; Geier, A.; Kettenbach, J.; Terracciano, L.; Weimann, R. Radiofrequency ablation suppresses distant tumour growth in a novel rat model of multifocal hepatocellular carcinoma. Clin. Sci. 2014, 126, 243–252.
- Napoletano, C.; Taurino, F.; Biffoni, M.; De Majo, A.; Coscarella, G.; Bellati, F.; Rahimi, H.; Pauselli, S.; Pellicciotta, I.; Burchell, J.M.; et al. RFA strongly modulates the immune system and anti-tumor immune responses in metastatic liver patients. Int. J. Oncol. 2008, 32, 481–490.
- Nobuoka, D.; Motomura, Y.; Shirakawa, H.; Yoshikawa, T.; Kuronuma, T.; Takahashi, M.; Nakachi, K.; Ishii, H.; Furuse, J.; Gotohda, D.; et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int. J. Oncol. 2011, 40, 63–70.
- Ali, M.Y.; Grimm, C.F.; Ritter, M.; Mohr, L.; Allgaier, H.-P.; Weth, R.; Bocher, W.O.; Endrulat, K.; Blum, H.E.; Geissler, M. Activation of dendritic cells by local ablation of hepatocellular carcinoma. J. Hepatol. 2005, 43, 817–822.
- Ji, L.; Gu, J.; Chen, L.; Miao, D. Changes of Th1/Th2 cytokines in patients with primary hepatocellular carcinoma after ultrasound-guided ablation. Int. J. Clin Exp. Pathol. 2017, 10, 8715–8720.
- Cho, Y.K.; Kim, J.K.; Kim, M.Y.; Rhim, H.; Han, J.K. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 2009, 49, 453–459.
- Wissniowski, T.T.; Hänsler, J.; Neureiter, D.; Frieser, M.; Schaber, S.; Esslinger, B.; Voll, R.; Strobel, D.; Hahn, E.G.; Schuppan, D.; et al. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003, 63, 6496–6500.
- Zerbini, A.; Pilli, M.; Penna, A.; Pelosi, G.; Schianchi, C.; Molinari, A.; Schivazappa, S.; Zibera, C.; Fagnoni, F.F.; Ferrari, C.; et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006, 66, 1139–1146.
- Zeng, Z.; Shi, F.; Zhou, L.; Zhang, M.-N.; Chen, Y.; Chang, X.-J.; Lu, Y.-Y.; Bai, W.-L.; Qu, J.-H.; Wang, C.-P.; et al. Upregulation of Circulating PD-L1/PD-1 Is Associated with Poor Post-Cryoablation Prognosis in Patients with HBV-Related Hepatocellular Carcinoma. PLoS ONE 2011, 6, e23621.
- Jing, X.; Zhou, Y.; Xu, X.; Ding, J.; Wang, F.; Wang, Y.; Wang, P. Dynamic changes of T-cell subsets and their relation with tumor recurrence after microwave ablation in patients with hepatocellular carcinoma. J. Cancer Res. Ther. 2018, 14, 40–45.
- Behm, B.; Di Fazio, P.; Michl, P.; Neureiter, D.; Kemmerling, R.; Hahn, E.G.; Strobel, D.; Gress, T.; Schuppan, D.; Wissniowski, T.T. Additive antitumour response to the rabbit VX2 hepatoma by combined radio frequency ablation and toll like receptor 9 stimulation. Gut 2014, 65, 134–143.
- Den Brok, M.H.M.G.M.; Sutmuller, R.P.M.; Van Der Voort, R.; Bennink, E.J.; Figdor, C.; Ruers, T.J.M.; Adema, G.J. In Situ Tumor Ablation Creates an Antigen Source for the Generation of Antitumor Immunity. Cancer Res. 2004, 64, 4024–4029.
- Shi, L.; Chen, L.; Wu, C.; Zhu, Y.; Xu, B.; Zheng, X.; Sun, M.; Wen, W.; Dai, X.; Yang, M.; et al. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin. Cancer Res. 2016, 22, 1173–1184.
- Iida, N.; Nakamoto, Y.; Baba, T.; Nakagawa, H.; Mizukoshi, E.; Naito, M.; Mukaida, N.; Kaneko, S. Antitumor Effect after Radiofrequency Ablation of Murine Hepatoma Is Augmented by an Active Variant of CC Chemokine Ligand 3/Macrophage Inflammatory Protein-1α. Cancer Res. 2010, 70, 6556–6565.
- Arihara, F.; Mizukoshi, E.; Kitahara, M.; Takata, Y.; Arai, K.; Yamashita, T.; Nakamoto, Y.; Kaneko, S. Increase in CD14+HLA-DR−/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol. Immunother. 2013, 62, 1421–1430.
- Han, G.; Berhane, S.; Toyoda, H.; Bettinger, D.; Elshaarawy, O.; Chan, A.W.H.; Kirstein, M.; Mosconi, C.; Hucke, F.; Palmer, D.; et al. Prediction of Survival Among Patients Receiving Transarterial Chemoembolization for Hepatocellular Carcinoma: A Response-Based Approach. Hepatology 2020, 72, 198–212.
- Han, J.W.; Yoon, S.K. Tissue-Resident Lymphocytes: Implications in Immunotherapy for Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020, 22, 232.
- Kim, M.J.; Jang, J.W.; Oh, B.S.; Kwon, J.H.; Chung, K.W.; Jung, H.S.; Jekarl, D.W.; Lee, S. Change in inflammatory cytokine profiles after transarterial chemotherapy in patients with hepatocellular carcinoma. Cytokine 2013, 64, 516–522.
- Kohles, N.; Nagel, D.; Jüngst, D.; Stieber, P.; Holdenrieder, S. Predictive value of immunogenic cell death biomarkers HMGB1, sRAGE, and DNase in liver cancer patients receiving transarterial chemoembolization therapy. Tumor Biol. 2012, 33, 2401–2409.
- Wang, W.; Chapman, N.M.; Zhang, B.; Li, M.; Fan, M.; Laribee, R.N.; Zaidi, M.R.; Pfeffer, L.M.; Chi, H.; Wu, Z.H. Upregulation of PD-L1 via HMGB1-Activated IRF3 and NF-kappaB Contributes to UV Radiation-Induced Immune Suppression. Cancer Res. 2019, 79, 2909–2922.
- Liao, J.; Xiao, J.; Zhou, Y.; Liu, Z.; Wang, C. Effect of transcatheter arterial chemoembolization on cellular immune function and regulatory T cells in patients with hepatocellular carcinoma. Mol. Med. Rep. 2015, 12, 6065–6071.
- Huang, M.; Wang, X.; Bin, H. Effect of Transcatheter Arterial Chemoembolization Combined with Argon-Helium Cryosurgery System on the Changes of NK Cells and T Cell Subsets in Peripheral Blood of Hepatocellular Carcinoma Patients. Cell Biochem. Biophys. 2015, 73, 787–792.
- Ayaru, L.; Pereira, S.P.; Alisa, A.; Pathan, A.A.; Williams, R.; Davidson, B.; Burroughs, A.K.; Meyer, T.; Behboudi, S. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J. Immunol. 2007, 178, 1914–1922.
- Guo, J.; Wang, S.; Han, Y.; Jia, Z.; Wang, R. Effects of transarterial chemoembolization on the immunological function of patients with hepatocellular carcinoma. Oncol. Lett. 2021, 22, 1–8.
- Park, H.; Jung, J.H.; Jung, M.K.; Shin, E.-C.; Ro, S.W.; Park, J.H.; Kim, D.Y.; Park, J.Y.; Han, K.-H. Effects of transarterial chemoembolization on regulatory T cell and its subpopulations in patients with hepatocellular carcinoma. Hepatol. Int. 2020, 14, 249–258.
- Xiong, B.; Feng, G.; Luo, S.; Liang, H.; Qiu, L.; Zheng, C.; Liu, X.; Zhou, G. Changes of CD4+ CD25+ regulatory T cells in peripheral blood in patients with hepatocellular carcinoma before and after TACE. J. Huazhong Univ. Sci. Technol. Med. Sci. 2008, 28, 645–648.
- Kalathil, S.G.; Lugade, A.A.; Miller, A.; Iyer, R.; Thanavala, Y. PD-1(+) and Foxp3(+) T cell reduction correlates with survival of HCC patients after sorafenib therapy. JCI Insight 2016, 1, e86182.
- Sangro, B.; Inarrairaegui, M.; Bilbao, J.I. Radioembolization for hepatocellular carcinoma. J. Hepatol. 2012, 56, 464–473.
- Fernandez-Ros, N.; Inarrairaegui, M.; Paramo, J.A.; Berasain, C.; Avila, M.A.; Chopitea, A.; Varo, N.; Sarobe, P.; Bilbao, J.I.; Dominguez, I.; et al. Radioembolization of hepatocellular carcinoma activates liver regeneration, induces inflammation and endothelial stress and activates coagulation. Liver Int. 2015, 35, 1590–1596.
- Seidensticker, M.; Powerski, M.; Seidensticker, R.; Damm, R.; Mohnike, K.; Garlipp, B.; Klopffleisch, M.; Amthauer, H.; Ricke, J.; Pech, M. Cytokines and 90Y-Radioembolization: Relation to Liver Function and Overall Survival. Cardiovasc. Interv. Radiol. 2017, 40, 1185–1195.
- Chew, V.; Lee, Y.H.; Pan, L.; Nasir, N.J.M.; Lim, C.J.; Chua, C.; Lai, L.; Hazirah, S.N.; Lim, T.K.H.; Goh, B.K.P.; et al. Immune activation underlies a sustained clinical response to Yttrium-90 radioembolisation in hepatocellular carcinoma. Gut 2019, 68, 335–346.




