Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luca Quartuccio | + 1902 word(s) | 1902 | 2021-09-02 08:17:59 | | | |
| 2 | Vicky Zhou | + 14 word(s) | 1916 | 2021-09-02 08:41:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Quartuccio, L. Antineutrophil Cytoplasmatic Antibody-Associated Vasculitis. Encyclopedia. Available online: https://encyclopedia.pub/entry/13816 (accessed on 08 February 2026).
Quartuccio L. Antineutrophil Cytoplasmatic Antibody-Associated Vasculitis. Encyclopedia. Available at: https://encyclopedia.pub/entry/13816. Accessed February 08, 2026.
Quartuccio, Luca. "Antineutrophil Cytoplasmatic Antibody-Associated Vasculitis" Encyclopedia, https://encyclopedia.pub/entry/13816 (accessed February 08, 2026).
Quartuccio, L. (2021, September 02). Antineutrophil Cytoplasmatic Antibody-Associated Vasculitis. In Encyclopedia. https://encyclopedia.pub/entry/13816
Quartuccio, Luca. "Antineutrophil Cytoplasmatic Antibody-Associated Vasculitis." Encyclopedia. Web. 02 September, 2021.
Copy Citation
Antineutrophil cytoplasmatic antibody (ANCA)-associated vasculitis (AAV) is a group of rare autoimmune diseases characterized by inflammation of the vascular wall. The pathogenesis of AAV is strongly associated with B cell-derived ANCAs; thus, Rituximab (RTX) has become a promising drug in the induction and maintenance treatment of AAV.
vasculitis
ANCA
rituximab
B cell
1. Introduction
Antineutrophil cytoplasmatic antibody (ANCA)-associated vasculitis (AAV) is a small-sized blood vessel vasculitis. AAV encompasses a heterogeneous group of rare autoimmune diseases represented by granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA) [1][2]. The terminology is linked to the presence of circulating autoantibodies, namely ANCAs, which are directed against the antigens found in the granules of neutrophils, most commonly either proteinase 3 (PR3) or myeloperoxidase (MPO) [3]. Typically, PR3-ANCA is detected in GPA (80–90% of patients), and MPO-ANCA is detected in MPA (60–85% of patients) [3]. In EGPA, the presence of ANCA shows more variability (30–60% of patients) and mainly involves MPO-ANCA [3]. AAV is a rare disease and in recent decades, several studies on its incidence and prevalence have been conducted, reporting a progressive worldwide increase [4]. Globally, the annual incidence ranges from 1.2 to 3.3 cases per 100,000 individuals, and the prevalence of AAV ranges from 4.6 to 42.1 cases per 100,000 individuals [4]. There is no clear gender predominance, though a slight male predominance among MPA compared to GPA has been reported [5][6]. Significant geographic differences have been reported among AAV subgroups, emphasising how the incidence of GPA and EGPA increases with latitude [7]. MPO-AAV and MPA are more common in Japan. PR3-AAV and GPA are more common in Europe [4]. ANCA specificity has a growing interest in the scientific community; in fact, it may fit better than clinical diagnosis for defining homogeneous groups of patients as well as for relapsing disease and clinical outcome [2].
Although AAV is a rare disease and its prevalence is geographically heterogeneous, recent studies concerning the healthcare burden of AAV reveal a high level of economic source consumption for the healthcare system [8][9]. Considering the wide spectrum of AAV organ manifestations, it is not surprising that the major cost component is the high rate of hospitalization. AAV can lead to a wide range of clinical manifestations; Table 1 shows possible multi-organ involvement. Involvement ranges from mild, such as ear, nose, and throat (ENT), to potentially life-threatening, such as alveolar haemorrhage [10].
Renal involvement is very common in GPA and MPA, especially at the onset of the disease [2]. Rapidly progressive glomerulonephritis with renal failure associated with proteinuria, microscopic haematuria, and hypertension can be the typical renal presentation [2]. Kidney biopsy typically reveals a pauci-immune focal necrotizing crescent glomerulonephritis [2]. Other histopathological features may include glomerular crescent or tubular intraepithelial infiltrates (acute inflammation) as well as glomerulosclerosis or interstitial fibrosis or tubular atrophy (chronic inflammation).
Validated scales to evaluate activity (Birmingham Vasculitis Activity Score (BVAS)) [11], damage (Vasculitis Damage Index (VDI) [12], and disease prognosis (Five-Factor Score (FFS)) [13] as well as a questionnaire about quality of life (AAV patient-reported outcomes (AAV-PRO)) [14], are extremely useful to support physicians in their choice of treatment [15].
The prognosis of AAV has greatly improved, and the 5-year survival rate has risen to around 70–80% over the past 40–50 years [16]. Several clinical factors influence the outcome, and the FFS can be applied to predict prognosis. Certainly, age and life-threatening disease at onset, for instance pulmonary-renal syndrome, influence the outcome [17]. The risk of end-stage renal disease (ESRD) is closely related with renal function at onset [18], and the findings of kidney biopsy correlate with the severity of renal involvement. The main causes of death in AAV patients are active disease and infections [19]. Additionally, among patients admitted to the intensive care unit for acute manifestations, the main causes of death are flares and infections [20][21].
Knowledge of ANCA specificity improves the classification of patients into distinct outcome categories. Many studies have focused on the sub-classification of AAV phenotypes based on different clusters (i.e., PR3-AAV, MPO-AAV, ANCA-negative). For instance, MPO-ANCA occurs in more than 80% of patients with isolated crescentic glomerulonephritis, whereas PR3-ANCA is present in more than 80% of patients with lung cavities or destructive ENT involvement [22]. Evidence of a higher risk of relapse has been found in PR3-AAV patients with renal involvement compared to non-PR3-AAV patients with renal involvement [23]. Patients with MPO-GPA show more frequently limited diseases with no severe organ involvement, a higher prevalence of subglottic stenosis, and fewer relapses than patients with PR3-GPA [22]. The highest mortality risk is observed in AAV patients with gastrointestinal (GI) and cardiovascular involvement [24]. ANCA-negative EGPA is more prone to cardiovascular involvement and experiencing higher mortality than ANCA-positive EGPA [24]. Nevertheless, up to 30% of AAV patients are ANCA-negative [24]. Several possible explanations are proposed, including the timing of ANCA testing, the presence of other pathogenic autoantibodies, the variable sensitivity of ANCA detection methods, and the presence of ANCA inhibitors that interfere with their identification.
Table 1. Clinical and laboratory characteristics of patients with antineutrophil cytoplasmatic antibody (ANCA)-associated vasculitis.
| Clinical Manifestations | MPA | GPA | EGPA |
|---|---|---|---|
| Constitutional symptoms | Fever, Weight Loss, Fatigue, Arthralgia, Myalgia | ||
| 55–80% | 70–100% | 30–50% | |
| Skin | Palpable Purpura, Nodules, Pseudourticarial Rash, Livedo Reticularis, Ulcers | ||
| 35–60% | 10–50% | 50–70% | |
| ENT | Infrequent | Frequent (60–80%): Destructive Sinusitis, Saddle-Nose Deformity, Crusting Rhinitis, Nasal Septum Deformity, Otitis Media | Allergic Rhinitis, Sinus Polyposis |
| Lung | Frequent (60–80%): Cough, Haemoptysis, Dyspnoea, Interstitial Lung Pattern, Alveolar Haemorrhage |
Frequent (60–80%): Non-Migratory Nodule or Infiltrates, Excavated Nodules, Bronchial And/or Subglottic Stenosis |
Asthma (Approximately 100%), Migratory Nodules or Infiltrates, Eosinophil Pleural Effusion |
| Kidney | Proteinuria, Haematuria, Renal Failure | ||
| Frequent (80%): Glomerulonephritis |
Frequent (60–80%): Glomerulonephritis |
Possible (20%) | |
| Neurologic | Mononeuritis Multiplex, Polyneuropathy, Cranial Nerves Disorders, Pachymeningitis | ||
| Possible (35%) | Possible (25%) | Frequent (65–75%) | |
| Heart | Myocarditis, Pericarditis, Ischemia | ||
| Possible (10–50%): From Asymptomatic to Cardiomyopathy |
|||
| Eye | Uveitis, Conjunctivitis, Episcleritis | ||
| Mono or Bilateral Proptosis, Orbital Tumour | |||
| Venous thrombosis | 7–8% | ||
| Laboratory | Increase ESR and CRP, Anaemia, Thrombocytosis | ||
| Eosinophilia | |||
| cANCA/PR3 | 10–20% | 80–90% | |
| pANCA/MPO | 60–85% | 0–10% | 30–60%, usually pANCA/MPO |
Legend: MPA, microscopic polyangiitis; GPA, granulomatosis with polyangiitis; EGPA, eosinophilic granulomatosis with polyangiitis; ENT, ear, nose and throat; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; ANCA, antineutrophil cytoplasmatic antibody; cANCA, cytoplasmic ANCA pattern; PR3, proteinase 3; pANCA, perinuclear ANCA pattern; MPO, myeloperoxidase.
2. Safety of Rituximab in AAV
Along with a better prognosis for AAV patients, the safety of long-term therapies has progressively become a primary focus of interest. The optimal balance between AAV therapy risks and benefits is a well-known and persistent challenge.
Infections and infusion reactions are the most common adverse events. The issue of the infections remains open since they still represent the most important cause of hospitalization and mortality, which is sometimes related to secondary hypogammaglobulinemia [29].
Hypogammaglobulinemia following RTX is not uncommon and more likely in patients with high GCs and CYC exposure and low IgG levels at baseline [30][31]. Hypogammaglobulinemia is typically defined as a serum IgG level below 600 mg/dL and can be further stratified as mild (400–599 mg/dL), moderate (200–399 mg/dL), and severe (0–199 mg/dL). Recommendations for the management of secondary hypogammaglobulinaemia due to B cell targeted therapies in autoimmune rheumatic diseases has recently been published although the strength of the recommendations was limited by the low quality of the evidence and the absence of randomized controlled trials [31]. Notably, immunoglobulin replacement can reduce the infection rate, but not the severe infection rate, in patients with a recurrence of infections [30]. Prophylaxis for Pneumocystis jirovecii during RTX therapy should be considered in all patients, both in the induction and maintenance regimen, and flu and pneumonia vaccination should be encouraged. In fact, the rate of infections can be lowered by trimethoprim–sulfamethoxazole in AAV undergoing RTX [32][33]. Additionally, RTX is considered a risk factor for poor outcomes in the case of SARS-CoV-2 infection [34][35][36]; thus, AAV patients who need RTX therapy should be recommended to undergo SARS-CoV-2 vaccination before RTX, if possible [34][35].
The incidence of infection with RTX is largely conditioned by the use of concomitant corticosteroids, previous treatments, and comorbidities [37]. The incidence of infectious complications with RTX was as high as with CYC, and this issue needs further investigation [38]. The concomitant use of corticosteroids together with lung comorbidity and diabetes are probably the main drivers for the risk of infections in AAV under RTX, and novel treatment strategies should be aimed to address the issue of sparing corticosteroids in the short and long term [39]. Several ongoing randomized controlled trials are aiming to optimize RTX dosage, possibly in combination with other drugs (CYC or belimumab or avacopan), and to minimize or possibly avoid glucocorticoids (Clinicaltrials.gov. NCT03942887; NCT03967925; NCT03920722; NCT032290456; NCT02749292; NCT02994927). In this regard, very recently, a Japanese phase 4, multicentre, open-label, randomized, noninferiority trial compared two corticosteroid regimens (reduced-dose prednisolone 0.5 mg/kg/day versus high-dose prednisolone 1 mg/kg/day) plus RTX 375 mg/m2/week, four doses in 140 patients with newly diagnosed AAV without severe glomerulonephritis or alveolar haemorrhage. In this trial, there was no difference in the primary endpoint, which was the remission rate at 6 months. Importantly, serious adverse events and, in particular, serious infections occurred at a significantly lower rate in the reduced-dose prednisolone arm [40]. Overall, positive results, if confirmed, will be of major value to improving the safety of the induction regimen with RTX.
Overall, the rate of infusion reactions was low (5%) [38][41] Infusion related-reactions (IRR) are usually mild to moderate, though fatal evolutions have been reported [38][41]. The most common IRR are fever, rash, itching, and headache [38][41]. More severe IRR includes angioedema, hypotension, and bronchospasm [38][41].
Late delayed neutropenia can usually be observed 6–8 months after RTX treatment, and it is more frequent in GPA (23%) than in lupus or rheumatoid arthritis [42]. Late-onset neutropenia can be observed in patients with a RTX maintenance regimen and usually recovers without treatment [43]. It is rarely associated with serious infections, which is different from early neutropenia, which is less frequent, but is possibly complicated by serious infections [42].
Data from the European Vasculitis Study Group (EUVAS) demonstrated a 2.8-fold incidence of an increased risk for non-melanoma skin cancer (NMSC) in AAV patients and a non-significant standardized incidence ratio (SIR) for non-NMSC (1.30) than general population expectations [44]. Recently, a propensity score-matched analysis of a nationwide study demonstrated that age, male sex, GPA sub-type, and CYC therapy was associated with cancer risk in AAV [45]. The malignancy risk in patients with AAV was lower in RTX-treated patients than in CYC-treated patients [46]. Notably, RTX treatment was not associated with an increased malignancy risk compared to the general population [46].
Despite the lack of head-to-head trials, retrospective studies supported that similar biologic RTX was as effective and safe as an originator in induction and remission maintenance in patients with AAV [47][48]. A recent systematic review comparing the four-dose (375 mg/m2 intravenously weekly) versus the two-dose (1000 mg intravenously biweekly) regimens in AAV did not find any differences for either efficacy or safety [49].
3. Conclusions
RTX plays an important role in AAV induction and maintenance therapy, especially in some subgroups of patients (Figure 1). After the introduction of CYC, which significantly improved the survival of AAV [50], the efficacy of RTX in AAV successfully addressed the issues of fertility preservation and the increased risk of malignancy under CYC. A maintenance therapy with RTX can decrease the rate of relapse and, as a consequence, the cumulative dose of corticosteroids. The optimal duration of RTX maintenance remains unknown, and further studies are required. The ANCA antibody seems to be a promising biomarker to guide RTX maintenance since an increased ANCA titre could reflect the incomplete B cell depletion and subclinical disease activity that may still require B-cell depletion [51]. RTX can be considered a long-term treatment for AAV with correctable side effects. Optimizing B cell-depleting therapy and steroid-sparing regimens is the next step towards further improvements in both the mortality rate and quality of life of AAV patients.
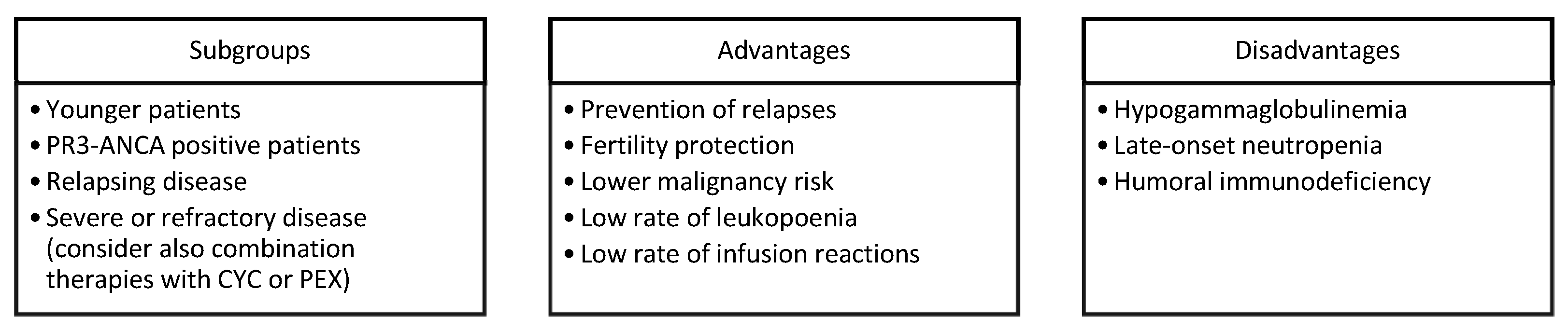
Figure 1. Major drivers, PROS, and CONS for choosing RTX in AVV. Legend: PR3, proteinase 3; CYC, cyclophosphamide; PEX, plasma exchange.
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11.
- Geetha, D.; Jefferson, J.A. ANCA-Associated Vasculitis: Core Curriculum 2020. Am. J. Kidney Dis. 2020, 75, 124–137.
- Salvador, F. ANCA Associated Vasculitis. Eur. J. Intern. Med. 2020, 74, 18–28.
- Berti, A.; Dejaco, C. Update on the Epidemiology, Risk Factors, and Outcomes of Systemic Vasculitides. Best Pr. Res. Clin. Rheumatol. 2018, 32, 271–294.
- Mohammad, A.J.; Jacobsson, L.T.H.; Mahr, A.D.; Sturfelt, G.; Segelmark, M. Prevalence of Wegener’s Granulomatosis, Microscopic Polyangiitis, Polyarteritis Nodosa and Churg-Strauss Syndrome within a Defined Population in Southern Sweden. Rheumatology 2007, 46, 1329–1337.
- Comarmond, C.; Pagnoux, C.; Khellaf, M.; Cordier, J.-F.; Hamidou, M.; Viallard, J.-F.; Maurier, F.; Jouneau, S.; Bienvenu, B.; Puéchal, X.; et al. Eosinophilic Granulomatosis with Polyangiitis (Churg-Strauss): Clinical Characteristics and Long-Term Followup of the 383 Patients Enrolled in the French Vasculitis Study Group Cohort. Arthritis Rheum. 2013, 65, 270–281.
- Watts, R.A.; Mahr, A.; Mohammad, A.J.; Gatenby, P.; Basu, N.; Flores-Suárez, L.F. Classification, Epidemiology and Clinical Subgrouping of Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitis. Nephrol. Dial. Transpl. 2015, 30 (Suppl. S1), i14–i22.
- Quartuccio, L.; Treppo, E.; Valent, F.; De Vita, S. Healthcare and Economic Burden of ANCA-Associated Vasculitis in Italy: An Integrated Analysis from Clinical and Administrative Databases. Intern. Emerg. Med. 2020, 16, 581–589.
- Ungprasert, P.; Koster, M.J.; Cheungpasitporn, W.; Wijarnpreecha, K.; Thongprayoon, C.; Kroner, P.T. Inpatient Epidemiology and Economic Burden of Granulomatosis with Polyangiitis: A 10-Year Study of the National Inpatient Sample. Rheumatology 2020, 59, 3685–3689.
- Quartuccio, L.; Bond, M.; Isola, M.; Monti, S.; Felicetti, M.; Furini, F.; Murgia, S.; Berti, A.; Silvestri, E.; Pazzola, G.; et al. Alveolar Haemorrhage in ANCA-Associated Vasculitis: Long-Term Outcome and Mortality Predictors. J. Autoimmun. 2020, 108, 102397.
- Mukhtyar, C.; Lee, R.; Brown, D.; Carruthers, D.; Dasgupta, B.; Dubey, S.; Flossmann, O.; Hall, C.; Hollywood, J.; Jayne, D.; et al. Modification and Validation of the Birmingham Vasculitis Activity Score (Version 3). Ann. Rheum. Dis. 2009, 68, 1827–1832.
- Exley, A.R.; Bacon, P.A.; Luqmani, R.A.; Kitas, G.D.; Gordon, C.; Savage, C.O.; Adu, D. Development and Initial Validation of the Vasculitis Damage Index for the Standardized Clinical Assessment of Damage in the Systemic Vasculitides. Arthritis Rheum. 1997, 40, 371–380.
- Guillevin, L.; Pagnoux, C.; Seror, R.; Mahr, A.; Mouthon, L.; Le Toumelin, P. French Vasculitis Study Group (FVSG) The Five-Factor Score Revisited: Assessment of Prognoses of Systemic Necrotizing Vasculitides Based on the French Vasculitis Study Group (FVSG) Cohort. Medicine 2011, 90, 19–27.
- Robson, J.C.; Dawson, J.; Doll, H.; Cronholm, P.F.; Milman, N.; Kellom, K.; Ashdown, S.; Easley, E.; Gebhart, D.; Lanier, G.; et al. Validation of the ANCA-Associated Vasculitis Patient-Reported Outcomes (AAV-PRO) Questionnaire. Ann. Rheum. Dis. 2018, 77, 1157–1164.
- Geetha, D.; Jin, Q.; Scott, J.; Hruskova, Z.; Hanouneh, M.; Little, M.A.; Tesar, V.; Seo, P.; Jayne, D.; Pagnoux, C. Comparisons of Guidelines and Recommendations on Managing Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Kidney Int. Rep. 2018, 3, 1039–1049.
- Flossmann, O.; Berden, A.; de Groot, K.; Hagen, C.; Harper, L.; Heijl, C.; Höglund, P.; Jayne, D.; Luqmani, R.; Mahr, A.; et al. Long-Term Patient Survival in ANCA-Associated Vasculitis. Ann. Rheum. Dis. 2011, 70, 488–494.
- Berden, A.E.; Ferrario, F.; Hagen, E.C.; Jayne, D.R.; Jennette, J.C.; Joh, K.; Neumann, I.; Noël, L.-H.; Pusey, C.D.; Waldherr, R.; et al. Histopathologic Classification of ANCA-Associated Glomerulonephritis. J. Am. Soc. Nephrol. 2010, 21, 1628–1636.
- Sinico, R.A.; Di Toma, L.; Radice, A. Renal Involvement in Anti-Neutrophil Cytoplasmic Autoantibody Associated Vasculitis. Autoimmun. Rev. 2013, 12, 477–482.
- Heijl, C.; Mohammad, A.J.; Westman, K.; Höglund, P. Long-Term Patient Survival in a Swedish Population-Based Cohort of Patients with ANCA-Associated Vasculitis. RMD Open 2017, 3, e000435.
- Kimmoun, A.; Baux, E.; Das, V.; Terzi, N.; Talec, P.; Asfar, P.; Ehrmann, S.; Geri, G.; Grange, S.; Anguel, N.; et al. Outcomes of Patients Admitted to Intensive Care Units for Acute Manifestation of Small-Vessel Vasculitis: A Multicenter, Retrospective Study. Crit. Care 2016, 20, 27.
- Demiselle, J.; Auchabie, J.; Beloncle, F.; Gatault, P.; Grangé, S.; Du Cheyron, D.; Dellamonica, J.; Boyer, S.; Beauport, D.T.; Piquilloud, L.; et al. Patients with ANCA-Associated Vasculitis Admitted to the Intensive Care Unit with Acute Vasculitis Manifestations: A Retrospective and Comparative Multicentric Study. Ann. Intensive Care 2017, 7, 39.
- Lionaki, S.; Blyth, E.R.; Hogan, S.L.; Hu, Y.; Senior, B.A.; Jennette, C.E.; Nachman, P.H.; Jennette, J.C.; Falk, R.J. Classification of Antineutrophil Cytoplasmic Autoantibody Vasculitides: The Role of Antineutrophil Cytoplasmic Autoantibody Specificity for Myeloperoxidase or Proteinase 3 in Disease Recognition and Prognosis. Arthritis Rheum 2012, 64, 3452–3462.
- Mahr, A.; Katsahian, S.; Varet, H.; Guillevin, L.; Hagen, E.C.; Höglund, P.; Merkel, P.A.; Pagnoux, C.; Rasmussen, N.; Westman, K.; et al. Revisiting the Classification of Clinical Phenotypes of Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis: A Cluster Analysis. Ann. Rheum. Dis. 2013, 72, 1003–1010.
- Cornec, D.; Gall, E.C.-L.; Fervenza, F.C.; Specks, U. ANCA-Associated Vasculitis—Clinical Utility of Using ANCA Specificity to Classify Patients. Nat. Rev. Rheumatol. 2016, 12, 570–579.
- Greco, A.; De Virgilio, A.; Rizzo, M.I.; Gallo, A.; Magliulo, G.; Fusconi, M.; Ruoppolo, G.; Tombolini, M.; Turchetta, R.; de Vincentiis, M. Microscopic Polyangiitis: Advances in Diagnostic and Therapeutic Approaches. Autoimmun. Rev. 2015, 14, 837–844.
- Furuta, S.; Iwamoto, T.; Nakajima, H. Update on Eosinophilic Granulomatosis with Polyangiitis. Allergol. Int. 2019, 68, 430–436.
- Greco, A.; Marinelli, C.; Fusconi, M.; Macri, G.F.; Gallo, A.; De Virgilio, A.; Zambetti, G.; de Vincentiis, M. Clinic Manifestations in Granulomatosis with Polyangiitis. Int. J. Immunopathol. Pharm. 2016, 29, 151–159.
- Pagnoux, C. Updates in ANCA-Associated Vasculitis. Eur. J. Rheumatol. 2016, 3, 122–133.
- Little, M.A.; Nightingale, P.; Verburgh, C.A.; Hauser, T.; De Groot, K.; Savage, C.; Jayne, D.; Harper, L.; European Vasculitis Study (EUVAS) Group. Early mortality in systemic vasculitis: Relative contribution of adverse events and active vasculitis. Ann. Rheum. Dis. 2010, 69, 1036–1043.
- Tieu, J.; Smith, R.M.; Gopaluni, S.; Kumararatne, D.S.; McClure, M.; Manson, A.; Houghton, S.; Jayne, D.R.W. Rituximab Associated Hypogammaglobulinemia in Autoimmune Disease. Front. Immunol. 2021, 12, 671503.
- Wijetilleka, S.; Jayne, D.R.; Mukhtyar, C.; Ala, A.; Bright, P.D.; Chinoy, H.; Harper, L.; Kazmi, M.A.; Kiani-Alikhan, S.; Li, C.K.; et al. Recommendations for the management of secondary hypogammaglobulinaemia due to B cell targeted therapies in autoimmune rheumatic diseases. Rheumatology 2019, 58, 889–896.
- Kronbichler, A.; Kerschbaum, J.; Gopaluni, S.; Tieu, J.; Alberici, F.; Jones, R.B.; Smith, R.M.; Jayne, D.R.W. Trimethoprim-Sulfamethoxazole Prophylaxis Prevents Severe/Life-Threatening Infections Following Rituximab in Antineutrophil Cytoplasm Antibody-Associated Vasculitis. Ann. Rheum. Dis. 2018, 77, 1440–1447.
- Monti, S.; Delvino, P.; Riboli, M.; Rebuffi, C.; Xoxi, B.; De Silvestri, A.; Montecucco, C. The role of Trimethoprim/sulfametoxazole in reducing relapses and risk of infec-tions in ANCA-associated vasculitis: A meta-analysis. Rheumatology 2021, 22, keab267.
- Sparks, J.A.; Wallace, Z.S.; Seet, A.M.; Gianfrancesco, M.A.; Izadi, Z.; Hyrich, K.L.; Strangfeld, A.; Gossec, L.; Carmona, L.; Mateus, E.F.; et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: Results from the COVID-19 Global Rheumatology Alliance physician registry. Ann. Rheum. Dis. 2021, 80, 1137–1146.
- Quartuccio, L.; Treppo, E.; Binutti, M.; Del Frate, G.; De Vita, S. Timing of Rituximab and Immunoglobulin Level Influence the Risk of Death for COVID-19 in ANCA-Associated Vasculitis. Rheumatology 2021, 60, 3476–3477.
- Benucci, M.; Quartuccio, L.; Li Gobbi, F.; Damiani, A.; Grossi, V.; Infantino, M.; Manfredi, M. Persistence of RT-PCR-SARS-CoV-2 Infection and Delayed Serological Response, as a Possible Effect of Rituximab According to the Hypothesis of Schulze-Koops et Al. Ann. Rheum. Dis. 2020.
- Charles, P.; Perrodeau, É.; Samson, M.; Bonnotte, B.; Néel, A.; Agard, C.; Huart, A.; Karras, A.; Lifermann, F.; Godmer, P.; et al. Long-Term Rituximab Use to Maintain Remission of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: A Randomized Trial. Ann. Intern. Med. 2020, 173, 179–187.
- Stone, J.H.; Merkel, P.A.; Spiera, R.; Seo, P.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.G.M.; St Clair, E.W.; Turkiewicz, A.; Tchao, N.K.; et al. Rituximab versus Cyclophosphamide for ANCA-Associated Vasculitis. N. Engl. J. Med. 2010, 363, 221–232.
- McClure, M.E.; Zhu, Y.; Smith, R.M.; Gopaluni, S.; Tieu, J.; Pope, T.; Kristensen, K.E.; Jayne, D.R.W.; Barrett, J.; Jones, R.B. Long-term maintenance rituximab for ANCA-associated vasculitis: Relapse and infection prediction models. Rheumatology 2021, 60, 1491–1501, PMID: 33141217; PMCID: PMC7937025.
- Furuta, S.; Nakagomi, D.; Kobayashi, Y.; Hiraguri, M.; Sugiyama, T.; Amano, K.; Umibe, T.; Kono, H.; Kurasawa, K.; Kita, Y.; et al. Effect of Reduced-Dose vs High-Dose Glucocorticoids Added to Rituximab on Remission Induction in ANCA-Associated Vasculitis: A Randomized Clinical Trial. JAMA 2021, 325, 2178–2187.
- Paul, F.; Cartron, G. Infusion-Related Reactions to Rituximab: Frequency, Mechanisms and Predictors. Expert. Rev. Clin. Immunol. 2019, 15, 383–389.
- Tesfa, D.; Ajeganova, S.; Hägglund, H.; Sander, B.; Fadeel, B.; Hafström, I.; Palmblad, J. Late-Onset Neutropenia Following Rituximab Therapy in Rheumatic Diseases: Association with B Lymphocyte Depletion and Infections. Arthritis Rheum. 2011, 63, 2209–2214.
- Tieu, J.; Smith, R.; Basu, N.; Brogan, P.; D’Cruz, D.; Dhaun, N.; Flossmann, O.; Harper, L.; Jones, R.B.; Lanyon, P.C.; et al. Rituximab for Maintenance of Remission in ANCA-Associated Vasculitis: Expert Consensus Guidelines. Rheumatology 2020, 59, e24–e32.
- Heijl, C.; Harper, L.; Flossmann, O.; Stücker, I.; Scott, D.G.I.; Watts, R.A.; Höglund, P.; Westman, K.; Mahr, A. European Vasculitis Study Group (EUVAS) Incidence of Malignancy in Patients Treated for Antineutrophil Cytoplasm Antibody-Associated Vasculitis: Follow-up Data from European Vasculitis Study Group Clinical Trials. Ann. Rheum. Dis. 2011, 70, 1415–1421.
- Choi, S.T.; Ahn, S.V.; Lee, P.H.; Moon, C.M. The Cancer Risk According to Three Subtypes of ANCA-Associated Vasculitis: A Propensity Score-Matched Analysis of a Nationwide Study. Semin. Arthritis Rheum. 2021, 51, 692–699.
- van Daalen, E.E.; Rizzo, R.; Kronbichler, A.; Wolterbeek, R.; Bruijn, J.A.; Jayne, D.R.; Bajema, I.M.; Rahmattulla, C. Effect of Rituximab on Malignancy Risk in Patients with ANCA-Associated Vasculitis. Ann. Rheum. Dis. 2017, 76, 1064–1069.
- Mittal, S.; Naidu, G.S.R.S.N.K.; Jha, S.; Rathi, M.; Nada, R.; Minz, R.W.; Sharma, K.; Dhir, V.; Jain, S.; Sharma, A. Experience with Similar Biologic Rituximab in 77 Patients of Granulomatosis with Polyangiitis-a Real-Life Experience. Clin. Rheumatol. 2021, 40, 645–651.
- Kwon, H.C.; Kim, M.K.; Song, J.J.; Park, Y.B.; Lee, S.W. Rituximab Biosimilar Prevents Poor Outcomes of Microscopic Polyangiitis and Granulomatosis with Polyangiitis as Effectively as Rituximab Originator. Yonsei. Med. J. 2020, 61, 712–719.
- Bénard, V.; Farhat, C.; Zarandi-Nowroozi, M.; Durand, M.; Charles, P.; Puéchal, X.; Guillevin, L.; Pagnoux, C.; Makhzoum, J.P. Comparison of Two Rituximab Induction Regimens for Antineutrophil Cytoplasm Antibody-Associated Vasculitis: Systematic Review and Meta-Analysis. ACR Open Rheumatol. 2021, 3, 484–494.
- Fauci, A.S.; Haynes, B.F.; Katz, P.; Wolff, S.M. Wegener’s Granulomatosis: Prospective Clinical and Therapeutic Experience with 85 Patients for 21 Years. Ann. Intern. Med. 1983, 98, 76–85.
- Terrier, B.; Pagnoux, C.; Perrodeau, É.; Karras, A.; Khouatra, C.; Aumaître, O.; Cohen, P.; Decaux, O.; Desmurs-Clavel, H.; Maurier, F.; et al. Long-Term Efficacy of Remission-Maintenance Regimens for ANCA-Associated Vasculitides. Ann. Rheum. Dis. 2018, 77, 1150–1156.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
02 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




