Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thomas Missimer | + 3027 word(s) | 3027 | 2021-08-17 03:50:06 | | | |
| 2 | Bruce Ren | -21 word(s) | 3006 | 2021-08-25 04:47:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Missimer, T. Brackish Water Reverse Osmosis Desalination. Encyclopedia. Available online: https://encyclopedia.pub/entry/13506 (accessed on 07 February 2026).
Missimer T. Brackish Water Reverse Osmosis Desalination. Encyclopedia. Available at: https://encyclopedia.pub/entry/13506. Accessed February 07, 2026.
Missimer, Thomas. "Brackish Water Reverse Osmosis Desalination" Encyclopedia, https://encyclopedia.pub/entry/13506 (accessed February 07, 2026).
Missimer, T. (2021, August 24). Brackish Water Reverse Osmosis Desalination. In Encyclopedia. https://encyclopedia.pub/entry/13506
Missimer, Thomas. "Brackish Water Reverse Osmosis Desalination." Encyclopedia. Web. 24 August, 2021.
Copy Citation
Brackish water desalination, using the reverse osmosis (BWRO) process, has become common in global regions, where vast reserves of brackish groundwater are found (e.g., the United States, North Africa).
brackish water reverse osmosis (BWRO) desalination
capital cost of BWRO
operating cost of BWRO
impacts of feedwater quality on cost
impacts of capacity on cost
1. Introduction
Many regions of the world have limited freshwater supplies to meet the combined demands of human consumption, agriculture, and industry [1]. Limitations on the development and use of fresh groundwater resources have led to assessments on the use potential of saline groundwater. Saline groundwater occurs in abundance in many global locations [2]. Brackish water, with total dissolved solids (TDS) less than about 8000 mg/L, is used as a water supply source in many regions, such as North Africa, central Saudi Arabia, Jordan, and others, in combination with desalination using the brackish water reverse osmosis process [3][4].
In the United States, there are extensive saline groundwater sources that contain total dissolved solid (TDS) concentrations between 1000 and 10,000 mg/L [5][6][7][8][9][10][11][12][13]. The volume of brackish groundwater that is available for use is quite large and geographically extensive. Saline (brackish) water use by county, in the United States in 2010, is shown in Figure 1. Brackish groundwater is used for irrigation in some regions, when the TDS is under 1500 mg/L, and the vegetation is tolerant to that salinity and the resulting soil salt buildup [14][15][16].
Brackish groundwater is also a source of feedwater for many brackish water reverse osmosis (BWRO) desalination plants that produce potable water [8][12][17][18]. In 2010, nearly 250 municipal membrane treatment facilities operated in the United States, and this grew to 406 in 2018 [18][19] (Figure 2). Of this number, 295 are BWRO facilities with capacities over 95 m3/d [16].
The largest concentration of BWRO desalination plants in the United States occurs in Florida [20][21]. Other states, such as Texas, are planning to add many additional BWRO facilities to those that are currently operating [22]. In Southern Florida, in 2019, 40 BWRO and 3 seawater reverse osmosis desalination plants operated, with a total capacity of 1.09 million m3/d [21] (Figure 3).
Because of the global growth rate in the use of brackish water desalination, there is great interest in the capital and operating costs of these facilities, but few data compilations have been published using actual data. One of the purposes of this research is to provide factual data associated with specific facilities and the methods of operation that contribute to the OPEX costs. The costs associated with a total of seven BWRO desalination water treatment facilities, based on cost per cubic meter (m³) in the Southwest Florida region of the United States of America, have been compiled, to allow detailed examination. In Southwest Florida, fresh source water supplies are becoming increasingly hard to develop because of the explosive population growth, impacts on the environment, pumping-induced saltwater intrusion, competition with the use of public or private wells, limited surface-water resources, and climate change issues. The combined results of these factors reduce the available freshwater resources for use and increase the demand for potable water.
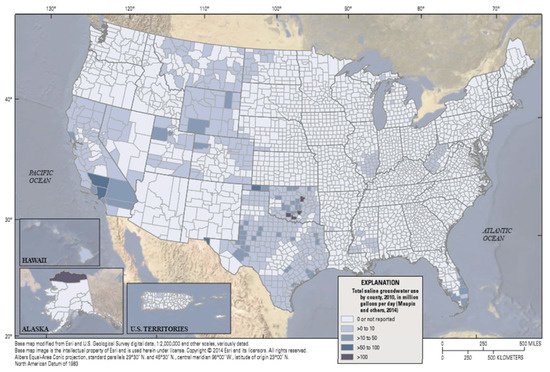
Figure 1. Total saline groundwater use in the United States by county in 2014 [23].
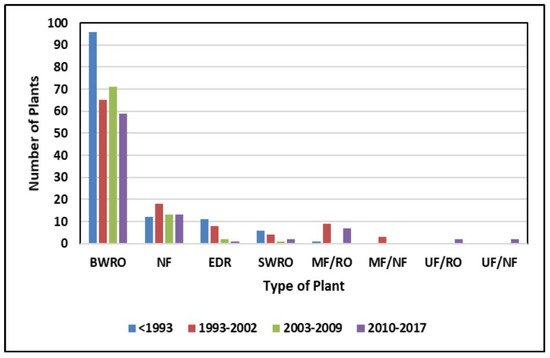
Figure 2. Number of desalination plants operating in the United States in 2010 [19].
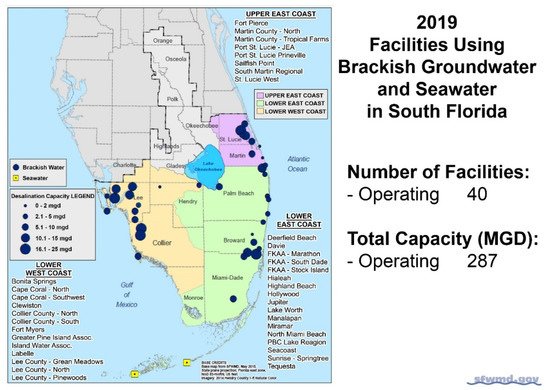
Figure 3. Locations and capacities of operating BWRO desalination plants in Southern Florida [21].
In addition to assessing the capital (CAPEX) and operating costs (OPEX) for the Southwest Florida facilities, an evaluation of BWRO costs was made, by comparing these detailed costs with the global data, to construct some basic graphs. These graphs can estimate the unit costs for various capacity plants and the impact of groundwater TDS on these costs. Where possible, the electrical cost was factored into the analysis. Most cost data for BWRO facilities have been estimated, rather than developed from the compilation of actual plant data. The inflation associated with the CAPEX and OPEX of the facilities, has been provided for the US facilities.
Most CAPEX and OPEX cost information on BWRO facilities operating costs is currently estimated using curves, models, or a set of assumptions [24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41]. It is a goal of this research to obtain real cost information for the existing BWRO plans, for comparison to the past estimates. Little consideration has been given to innovations in real plant operations, which reduce electrical usage and the overall cost of treatment. Some examples of innovative cost control measures include the blending of raw water with treated water, to raise the pH and to reduce the cost to the consumer; the use of energy recovery devices in BWRO; the blending of the raw water with some limited freshwater, to reduce the salinity of the feedwater (where available); and the implementation of specialized maintenance techniques that extend the lifetime of the membranes, from 5 up to 15 years (e.g., City of Cape Coral).
2. CAPEX Cost Variation
The CAPEX costs for BWRO facilities vary in the extreme, based on what is included in the project and land costs. The highest cost documented value of over USD 2947/m3 is the North City of Cape Coral, Florida facility. Based on the consumer price index over the years, since the construction of the project, the 2020 cost would be about USD 4067. The detailed breakdown of this cost shows that a large urban land purchase was required to build the BWRO facility, and to accommodate other utility infrastructure at the same location. The building was sized to meet the buildout capacity for the service area of the plant. Also, spare pumps were purchased in addition to other equipment that was needed to provide operational security. The raw water contained hydrogen sulfide, which had to be removed before the treated water could be discharged into the distribution system, so a degassing system had to be designed and constructed. The concentrate disposal for the plant was a deep-well injection, so that the cost was also included in the CAPEX. The Cape Coral North facility is an excellent example of why using cost curves to estimate CAPEX, for new BWRO facilities, can produce inaccurate estimates. Many projects involving BWRO design and construction also include multiple components that cannot be easily separated from the primary project goal. Commonly, distribution system improvements are also contained with these project budgets (e.g., storage tanks, pumping stations, and pipeline improvements). The use of cost curves for the estimation of strictly the BWRO component of a project is reasonable, if it can be separated from the other components of the project.
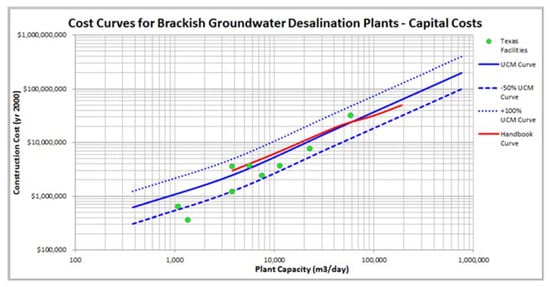
Perhaps the most consistent data set on BWRO CAPEX is that from Texas. These projects had a rather narrow set of goals and objectives that follow the general trend of reducing CAPEX/m3 with increasing plant capacity. The one possible exception is the largest capacity plant in El Paso (Figure 4). This facility uses a high-pressure deep injection well system for concentrate disposal.
3. OPEX Cost Variation
Some important general observations can be made in analyzing the complied OPEX data from various BWRO facilities. First, the local electrical cost is one of the key factors that controls BWRO treatment costs in facilities that treat raw water, within a TDS range of 2000 to 8000 mg/L, and do not have major pretreatment and concentrate disposal challenges. The OPEX costs for BWRO facilities in Florida are generally higher than in Texas, because the average electrical cost is about USD 0.6/kWh in Texas versus USD 0.125/kWh in Florida. The Florida rate is primarily for residential electric use, and industrial or utility rates are lower based on the negotiated rates, considering factors such as interruptible power (on-site generators) and peak load reduction. Most BWRO facilities in Florida must also post-treat the potable water to remove hydrogen sulfide. The international facility costs are lower, which could result from various types of subsidies, or other unknown reasons. At locations where the pretreatment costs only include an antiscalant and acid to reduce the pH, the costs are generally lower. Where any additional control of the substances in the raw water is required, such as silica, iron, or manganese, the costs can be quite high compared to the pretreatment that is used to solely control calcium carbonate scaling.
In general, the cost for BWRO plants capacities, ranging from 10,000 to 70,000 m3/d, is 0.39 to 0.66 USD/m3 based on the time when the data were collected. These data are not corrected for inflation, because the individual utilities do not raise the consumer rates annually and may lag behind real cost recovery. A graph showing all of the compiled OPEX data based on plant capacity (m3/d) versus cost in USD/m3 is given in Figure 5. Note that there is extreme scatter when plotting all of the data that were collected. The variations are caused primarily by the differences in energy costs, pretreatment of the feedwater, and post-treatment of the finished water. There is no distinct pattern of reducing unit costs with increasing plant capacity for all of the data combined. Still, the trend line shows a negative slope, indicating a reduction in OPEX as the capacity increases. The Texas data show a tighter fit to the capacity versus cost scaling line. A second plot of the plant capacity in m3/d versus the cost per day in USD produces less scatter, and a trend line can be drawn with a better correlation (Figure 6). Since the slope of the line is less than one-to-one, as shown in the regression equation, the scaling factor that indicates a lower unit cost as the capacity increase is again confirmed.
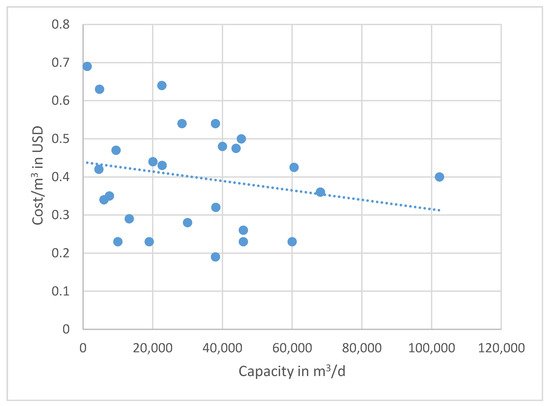
Figure 5. U.S. and global OPEX cost for various plant capacities. Note the extreme scatter of data based on the arithmetic plot. The R2 value of the trend line is poor, with the data having a standard deviation of 0.13 m2/d, but shows a reducing unit cost as capacity increases. Note that these costs are not corrected for local inflation, which may not directly correlate with increased costs to consumers (e.g., foreign water subsidies).
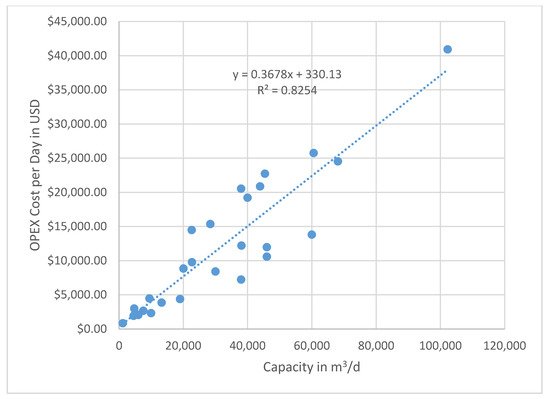
Figure 6. Plot of capacity versus total OPEX cost/day. Note that this plot shows a better fit to the trend line with a high R2 value. The equation for the trend line has a slope of less than one-to-one, which is indicative of a lower unit cost with a larger capacity. Therefore, scaling of capacity is an important economic factor. Note that these costs are not corrected for local inflation, which may not directly correlate with increased costs to consumers (e.g., foreign water subsidies).
4. Impacts of Energy Recovery Systems in BWRO
In the past, energy recovery systems were not considered to be effective in cost savings in BWRO plants, because of the low operating pressure, high recovery, and the use of blending with raw water. However, many BWRO plants are now being designed with energy recovery systems or retrofitted with these systems.
An evaluation of two types of energy recovery systems for a BWRO plant showed that both turbocharger and isobaric systems might save electric energy costs, especially when the conversion rate is <84% [43][44][45]; as the conversion rate declines, the electrical energy saving increases [46]. The key method in deciding on the use of energy recovery systems on BWRO plants is to conduct a complete life cycle analysis (LCA) [47].
A large number of BWRO facilities exhibit long-term increases in feed water salinity [48][49][50][51][52]. The use of energy recovery systems to mitigate electric energy costs increases as the feedwater salinity increases, which should be considered. This would potentially mitigate higher energy consumption, to treat higher TDS feedwater. Some recent research on the use of energy recovery in BWRO has suggested integrating a supercapacitor with capacitive deionization into the process [53].
5. Impacts of Feedwater Chemistry Issues That Can Potentially Affect the Economics of Brackish-Water Desalination
Feedwater chemistry can significantly impact BWRO costs if additional pretreatment is required to prevent membrane scaling and fouling [54]. In BWRO, the most common problem is scaling and not fouling, although some fouling has been reported at the City of Cape Coral North BWRO facility. There are four common types of scaling in BWRO plants, which are impacted by the feedwater chemistry. This includes scaling with calcium carbonate, calcium sulfate, iron, and silica [55].
Calcium carbonate scaling is commonly controlled using standard pretreatment methods, as described in this paper at the City of Cape Coral facilities. The pH of the inflow water is lowered using acid, and a polyphosphate or polyacrylate is added. Some recent research has been conducted on the use of polyaspartic acid as a pretreatment additive [56]. Unless the hardness of the feedwater is exceptionally high, the standard pretreatment process that is used to control calcium carbonate scaling does not add a higher cost to BWRO desalination. In feedwater with a very high hardness, the additional concentration of acid required could increase OPEX cost.
The control of calcium sulfate scaling (gypsum) can be considerably more complex [57][58]. The closer to saturation that occurs in the feedwater, the greater the difficulty of the pretreatment. A zwitterionic coating on the thin-film composite membranes has been suggested as a means for slowing gypsum scaling [59]. The addition of carboxymethyl cellulose in the feed may also reduce the rate of scaling [60]. The use of sulfuric acid to lower the pH is not recommended, but hydrochloric acid is more effective and does not add additional sulfate to the feedwater. However, hydrochloric acid is more expensive and tends to increase the treatment costs. When the feedwater chemistry contains a high relative concentration of sulfate-to-chloride ratio, the potential for scaling rises and increases the potential for gypsum scaling, the recovery using the RO process must be reduced, thereby significantly increasing the treatment cost [61]. In certain cases, it is more effective to treat this type of feedwater using electrodialysis or electrodialysis reversal if the overall TDS concentration is not too high [62][63][64]. In Florida, this issue occurs in Sarasota County.
The control of iron scaling in BWRO is commonly mitigated by the reduction in the feedwater pH, similar to calcium carbonate. However, if the dissolved iron concentration is too high or the feedwater varies between anoxic and oxic, the iron must be removed at considerable expense. In this case, it may be necessary to add a tray aerator, add a coagulant polymer, and then use a plate settler to remove the iron [65]. An alternative would be to use chlorine dioxide, a plate settler, micro-sand filtration, and then an oxidant remove stage to remove the iron [65]. Therefore, if the dissolved iron must be removed, the BWRO desalination process rises in cost.
Perhaps the most difficult potential scaling issue is that with silica. Most natural groundwater sources do not have high silica concentrations, but in aquifers with elevated temperatures (geothermal), silica concentrations can approach saturation or they can contain silica colloids. Because the scaling of silica on BWRO membranes may not be able to be removed, it is quite important that it be prevented from occurring. Two factors seem to dictate the scaling of silica on the membrane, which include the initial concentration in the feedwater (combined dissolved and colloids) and the surface condition of the membranes [66][67]. The surface electrostatic charge and the occurrence of certain organic materials can either accelerate or inhibit silica deposition. The typical pretreatment methods to prevent scaling are pH adjustment or to add an antiscalant solution [68][69]. These methods are common and do not generally add significant costs to the pretreatment. However, if pretreatment of the feedwater is required, then the costs can become quite high. Expensive pretreatment techniques, such as electrocoagulation, can be used to remove the silica from the brackish water before primary membrane treatment [70].
6. Impacts of Zero Liquid Discharge on the Economics of BWRO
Disposal of the concentrate after the BWRO process has also become a potentially large cost factor within the interior facility locations, where surface disposal into the ocean or the use of deep injection wells is not possible. In some locations, the use of zero liquid discharge (ZLD) is the only means of concentrate disposal. Within the realm of seawater desalination, the use of ZDL for large-capacity plants is likely a myth [71]. In small- to medium-capacity BWRO systems, ZLD is feasible, but requires a means of salt disposal, which causes increased costs for both the additional energy for treatment and the solid waste disposal [72][73][74]. A number of methods have been proposed to lower the energy consumption and costs for ZLD, by combining various membrane and thermal processes [75][76][77][78][79][80]. All of the methods, either used or designed to date, cause a major increase in power consumption, resulting in higher water production costs [81]. Perhaps mitigation methods could be used to co-locate inland BWRO plants, where a number of industries require very-high-quality water for makeup water to produce steam or where valuable metals could be extracted from the waste stream.
7. Comparative Costs between BRWO and Seawater RO (SWRO) Costs
Over the past decade, the cost of SWRO has steadily decreased, based on the common use of energy recovery devices and the scale factor that is associated with the design and construction of very-large-capacity facilities [38]. The OPEX cost of some seawater plants has now fallen below BWRO OPEX costs for some of the larger facilities, in the 100,000 to 1,000,000 m3/d range. A key difference between the SWRO and BWRO facilities is the chemistry differences in the raw water supply, and the ability to design and construct very-large-capacity SWRO plants above 200,000 m3/d. The issue of concentrate disposal is another factor favoring a lower seawater desalination cost, where the disposal is back into the ocean. While equivalent-capacity plants will continue to show higher OPEX costs for BWRO, the trend will continue to reduce SWRO costs as the capacities grow. It is interesting to note that the use of seawater from groundwater sources should significantly reduce pretreatment, due to the lack of organic carbon in groundwater. However, the possible occurrence of hydrogen sulfide in the feedwater will necessitate its removal after membrane treatment, and thereby could cause an increase in the overall treatment cost.
References
- United Nations Educational, Scientific and Cultural Organization (UNESCO). Water a Shared Responsibility: The United Nations Water Development Report 2; Berghahn Books: New York, NY, USA, 2007.
- van Weert, F.; van der Gun, J.; Reckman, J. Global Overview of Saline Groundwater Genesis; International Groundwater Assessment Centre Report GP 2009-1; Internationsl Groundwater Resources Assessment Center: Utrecht, The Netherlands, 2009.
- BRL Ingenerie and DHV Consultants. Seawater and Brackish-Water Desalination in the Middle East, North Africa, and Central Asia, A Review of Key Issues and Experience in Six Countries; Final Report for the World Bank; World Bank: Paris, France, 2004.
- Afonso, M.D.; Jaber, J.O.; Mohsen, M.S. Brackish groundwater treatment by reverse osmosis in Jorden. Desalination 2004, 164, 157–171.
- Feth, J.H. Selected References on Saline Ground-Water Resources of the United States; U.S. Geological Survey: Reston, VA, USA, 1965. Available online: https://pubs.er.usgs.gov/publication/cir499 (accessed on 10 May 2021).
- Bush, P.W.; Ulery, R.L.; Rittmaster, R.L. Dissolved-Solids Concentrations and Hydrochemical Facies in Water of the Edwards-Trinity Aquifer System, West Central Texas; U.S. Geological Survey Water-Resources Investigation Report 93–4126. 1994. Available online: https://pubs.er.usgs.gov/publication/wri934126 (accessed on 10 June 2021).
- Busby, J.F.; Kimball, B.A.; Downey, J.S.; Peter, K.D. Geochemistry of Water in Aquifers and Confining Units of the Northern Great Plains in Parts of Montana, North Dakota, South Dakota, and Wyoming; U.S. Geological Survey Professional Paper 1402–F. 1995. Available online: https://pubs.er.usgs.gov/publication/pp1402F (accessed on 10 June 2021).
- AndrowskiJ, J.; Springer, A.; Acker, T.; Manone, M. Wind-powered desalination—An estimate of saline groundwater in the United States. J. Am. Water Res. Assoc. 2011, 47, 93–101.
- Anning, D.W.; Bauch, N.J.; Gerner, S.J.; Flynn, M.E.; Hamlin, S.N.; Moore, S.J.; Schaefer, D.H.; Anderholm, S.K.; Spangler, L.E. Dissolved Solids in Basin-Fill Aquifers and Streams in the Southwestern United States (ver. 1.1, 22 March 2010). U.S. Geological Survey Scientific Investigations Report 2006–5315. 2010. Available online: https://pubs.er.usgs.gov/publication/sir20065315 (accessed on 10 June 2021).
- Thiros, S.A.; Paul, A.P.; Bexfiled, L.M.; Anning, D.W. The Quality of Our Nation’s Waters—Water Quality in Basin-Fill Aquifers of Southwestern United States: Arizona, California, Colorado, Nevada, New Mexico, and Utah, 1993–2009; US Geological Survey: Reston, VA, USA, 2014.
- McMahon, P.B.; Böhlke, J.K.; Dahm, K.G.; Parkhurst, D.L.; Anning, D.W.; Stanton, J.S. Chemical considerations for an updated national assessment of brackish groundwater resources. Groundwater 2015, 54, 464–475.
- Stanton, J.S.; Anning, D.W.; Brown, C.J.; Moore, R.B.; McGuire, P.B.; Degnan, J.R. Brackish Groundwater in the United States; U.S. Geological Survey Professional Paper 1833; U.S. Geological Survey: Reston, VA, USA, 2017.
- Qi, S.L.; Harris, A.C. Geochemical Database for the Brackish Groundwater Assessment of the United States; U.S. Geological Survey: Reston, VA, USA, 2017.
- Lunin, J.; Gallatin, M.H.; Bower, C.A.; Wilcox, L.V. Use of Brackish Water for Irrigation in Humid Regions; U.S. Department of Agriculture, Agricultural Research Service, Agriculture Information Bulletin No. 213. 1960. Available online: https://naldc.nal.usda.gov/download/CAT87210399/PDF#:~:text=brackish%20water%20for%20irrigation.%20If%20soils%20have%20been,be%20used%20for%20the%20crop%20that%20is%20being (accessed on 14 June 2021).
- NRS Engineering Water Solutions. Guidance Manual for Brackish Groundwater Desalination in Texas; Texas Water Development Board: Austin, TX, USA, 2008. Available online: http://www.twdb.texas.gov/publications/reports/contractedreports/doc/0604830581_BrackishDesal.pdf (accessed on 14 June 2021).
- Bauder, T.A.; Waskom, R.M.; Sutherland, P.L.; Davis, J.G. Irrigation Water Quality Criteria; Colorado State University Extension Fact Sheet 0.506; Colorado State University: Fort Collins, CO, USA, 2014; Available online: http://extension.colostate.edu/topic-areas/agriculture/irrigation-water-quality-criteria-0-506/ (accessed on 20 May 2021).
- National Research Council. Desalination: A National Perspective; The National Academies Press: Washington, DC, USA, 2008.
- Mickley, M. US municipal desalination plants—Number, types, locations, sizes, and concentrate management practices. J. Desal. Water Reuse 2012, 4, 44–51.
- Mickley, M. Updated and Extended Survey of U.S. Municipal Desalination Plants; Desalination and Water Purification Research and Development Program Report No. 27; U.S. Department of Interior, Bureau of Reclamation, Technical Service Center: Denver, CO, USA, 2018.
- Florida Department of Environmental Protection (FDEP). Desalination in Florida: Technology, Implementation, and Environmental Issues; Florida Department of Environmental Protection, Division of Water Resource Management: Tallahassee, FL, USA, 2010. Available online: https://ufdimages.uflib.ufl.edu/UF/00/10/82/00001/desalination-in-florida-report.pdf (accessed on 20 May 2021).
- South Florida Water Management District (SFWMD). Facilities Using Brackish Groundwater and Seawater in South Florida; South Florida Water Management District: West Palm Beach, FL, USA, 2019. Available online: https://www.sfwmd.gov/sites/default/files/2019 (accessed on 20 May 2021).
- Nicot, J.-P.; Walden, S.; Greenlee, L.; Els, J. A Desalination Database for Texas; Report Prepared for the Texas Water Development Board; Texas Bureau of Economic Geology: Austin, TX, USA, 2006; Available online: www.beg.utexas.edu/files/content/beg/research/water-energy-nexus/Final%20Report_R1_1.pdf (accessed on 20 May 2021).
- Maupin, M.A.; Kenny, J.F.; Hutson, J.S.S.; Lovelace, J.K.; Barber, N.L.; Linsey, K.S. Estimated Use of Water in the United States in 2010; U.S. Geological Survey Circular 1405; Reston, VA, USA, 2014. Available online: https://pubs.er.usgs.gov/publication/cir1405 (accessed on 15 June 2021).
- U.S. Department of Interior, Office of Saline Water. A Standardized Procedure for Costs of Saline Water Conversion; U.S. Department of Interior, Office of Saline Water: Washington, DC, USA, 1956.
- U.S. Department of the Interior, Office of Saline Water. Guidelines for Uniform Presentation of Desalting Cost Estimates; U.S. Department of Interior, Office of Saline Water: Washington, DC, USA, 1967.
- U.S. Office of Water Research and Technology. Desalting Handbook for Planners; U.S. Office of Water Research and Technology: Washington, DC, USA, 1972.
- U.S. Office of Water Research and Technology. Desalting Handbook for Planners, 2nd ed.; U.S. Office of Water Research and Technology: Washington, DC, USA, 1979.
- Leitner, G.M. International Desalination Association Desalting Costs Program, Brackish and Seawater; International Desalination Association: Topsfield, MA, USA, 1990.
- Chapman-Wilbert, M. The Desalting and Water Treatment Membrane Manual: A Guide to Membranes for Municipal Water Treatment, 2nd ed.; Water Treatment and Technology Program Report No. 29; U.S. Department of Interior, Bureau of Reclamation: Denver, CO, USA, 1998.
- U.S. Department of Interior, Bureau of Reclamation. Water Treatment Cost Estimation Routine; U.S. Department of Interior, Bureau of Reclamation: Denver, CO, USA, 1999.
- International Atomic Energy Agency. Desalination Economic Evaluation Program (DEEP); International Atomic Energy Agency: Vienna, Austria, 2000; Available online: https://www.iaea.org/publications/6049/desalination-economic-evaluation-program-deep (accessed on 10 July 2021).
- Moch, I.M.; Chapman, D.; Steward, D. WTCost—A computerized water treatment cost estimating program. In Proceedings of the International Desalination Association International Conference on Desalination and Water Reuse, Lanaca, Cyprus, 6–8 December 2004.
- Watson, I.; Morin, O.J.; Henthorne, L. Desalting Handbook for Planners, 3rd ed.; Desalination and Water Purification Research and Development Program Report No. 72; U.S. Department of Interior, Bureau of Reclamation: Denver, CO, USA, 2003.
- Adham, S.; Kumar, M.; Pearce, B. Development of a Model for Brackish and Reclaimed Water Membrane Desalination Costs; Desalination Research and Innovation Partnership (DROIP): Washington, DC, USA, 2004.
- Middle East Desalination Research Center (MEDRC). International Desalination Conference on Desalination Costing; Middle East Desalination Research Center: Muscat, Oman, 2004.
- Reddy, K.V.; Ghaffour, N. Over of the cost of desalinated water and costing methodologies. Desalination 2007, 205, 340–353.
- Montgomery Water & Harza. Brackish Ground Water Desalination Treatment Process Evaluation & Cost Model Development/Guidance Tool; Final Report, Report Prepared for the City of San Diego; Montgomery Water & Harza: San Diego, CA, USA, 2008.
- Moch, I.; Querns, W.R.; Stewart, D. Modeling the Capital and Operating Costs of Thermal Desalination Processes Utilizing a Recently Developed Computer Program that Evaluates Membrane Desalting, Electrodialysis, and Ion Exchange Plants; U.S. Department of Interior, Bureau of Reclamation, Technical Service Center, Denver Federal Center: Denver, CO, USA, 2008.
- Huehmer, R. Cost modeling of desalination systems. In Proceedings of the International Desalination Association World Conference on Desalination and Reuse, Perth, Australia, 4–9 September 2011.
- Frenkel, V. Consideration for the Co-Siting of Desalination Facilities with Municipal and Industrial Facilities; Final Project Report and Decisions Tool; WateReuse Research Foundation: Alexandria, VA, USA, 2012.
- Ghaffour, N.; Missimer, T.M.; Amy, G.L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination 2013, 9, 197–207.
- Texas Water Development Board. Uniform Costing Model User’s Guide; Texas Water Development Board: Austin, TX, USA, 2018.
- Al-Karaghouli, A.; Kazmerski, L.L. Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renew. Sustain. Energy Rev. 2013, 24, 343–356.
- Zarzo, D.; Campos, E.; Terrero, P. Spanish experience in desalination agriculture. Desalin. Water Treat. 2012, 51, 53–66.
- Chaudhry, S. Unit Cost of Desalination; California Desalination Task Force, California Energy Commission: Sacramento, CA, USA, 2003.
- Drak, A.; Adato, M. Energy recovery consideration in brackish water desalination. Desalination 2014, 239, 34–39.
- Martin, J.; Eisberg, D. Brackish Water Desalination—Energy and Cost Considerations. 2014. Available online: www.energyrecovery.com/wp-content/uploads/2014/12/ (accessed on 15 July 2021).
- Missimer, T.M. Water Supply Development, Aquifer Storage and Concentrate Disposal for Membrane Water Treatment Facilities, 2nd ed.; Methods in Water Resources Evaluation Series No. 1; Schlumberger Media: Sugar Land, TX, USA, 2009.
- Drendal, R.; Kinzli, K.D.; Koebel, A.; Missimer, T.M. Management of brackish water desalination systems using long-term monitoring of feed water quality. Desalin. Water Treat. 2016, 57, 16209–16219.
- Mead, E.; Victory, J.; Missimer, T.M. Changes in feed water salinity with pumping in wellfields used to supply a brackish water RO facility at the City of Fort Myers, Florida. Desalin. Water Treat. 2020, 171, 1–12.
- Schroeder, D.W.; Thomson, A.; Missimer, T.M. Characterization change of the production aquifer affects the successful design and operation of a brackish-water reverse osmosis plant over the lifespan of the facility, The Town of Jupiter, Florida. Desalin. Water Treat. 2021, 227, 1–15.
- Schroeder, D.W.; Missimer, T.M. Production aquifer water salinity change impacts on brackish-water reverse osmosis desalination facility process design: The City of Clewiston, Florida. Desalin. Water Treat. 2021.
- Chen, Y.-W.; Chen, J.-F.; Lin, C.-H.; Hou, C.-H. Integrating a supercapacitor with capacitive deionization for direct energy recovery from the desalination of brackish water. Appl. Energy 2019, 252, 113417.
- Zhao, S.; Liao, Z.; Fane, A.; Li, J.; Tang, C.; Zheng, C.; Liu, J.; Kong, L. Engineering antifouling reverse osmosis membranes: A review. Desalination 2021, 499, 114857.
- Honarparvar, S.; Zhang, X.; Chen, T.; Alborzi, A.; Afroz, K.; Reible, D. Frontiers of membranes desalination processes for brackish water treatment: A review. Membranes 2021, 11, 246.
- Pramanik, B.K.; Gao, Y.; Fan, L.; Roddick, F.A.; Liu, Z. Antiscaling effect of polyaspartic acid and its derivative for RO membranes used for saline wastewater and brackish water desalination. Desalination 2017, 404, 224–229.
- Sweity, A.; Zere, T.R.; David, I.; Bason, S.; Oren, Y.; Ronen, Z.; Herzberg, M. Side effects of antiscalants on biofouling of reverse osmosis membranes in brackish water desalination. J. Membr. Sci. 2015, 481, 172–187.
- Rabizadeh, T.; Morgan, D.J.; Peacock, C.L.; Benning, L.G. Effectiveness of green additives vs poly (acrylic acid) in inhibiting calcium sulfate dihydrate crystallization. Ind. Eng. Chem. Res. 2019, 58, 1561–1569.
- Jaramillo, H.; Boo, C.; Hashmi, S.M.; Elimelech, M. Zwitterionic coating on thin-film composite membranes to delay gypsum scaling in reverse osmosis. J. Membr. Sci. 2021, 618, 118568.
- Yu, W.; Song, D.; Li, A.; Yang, H. Control of gypsum-dominated scaling in reverse osmosis system using carboxymethyl cellulose. J. Membr. Sci. 2019, 577, 20–30.
- Rahmawati, K.; Ghaffour, N.; Aubry, C.; Amy, G.L. Boron removal efficiency from Red Sea water using different SWRO/BWRO membranes. J. Membr. Sci. 2012, 423–424, 522–529.
- Katz, W.E. The electrodialysis reversal (EDR) process. Desalination 1979, 28, 31–40.
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174.
- Turek, M.; Dydo, P. Electrodialysis reversal of calcium sulphate and calcium carbonate supersaturated solution. Desalination 2003, 158, 91–94.
- Missimer, T.M.; Watson, I.; Maliva, R.G.; Ghafour, N.; Dehwah, A.H.A.; Woolschlager, J.; Hegy, M. Impacts of natural pore-water an offshore aquifer chemistry on the operation and economics of some subsurface intake types for SWRO plants. Desalin. Water Treat. 2018, 132, 1–9.
- Rathinam, K.; Abraham, S.; Oren, Y.; Schwahn, D.; Petry, W.; Kaufman, Y.; Kasher, R. Surface-induced silica scaling during brackish water desalination: The role of surface charge and specific chemical groups. Environ. Sci. Technol. 2019, 53, 5202.
- Lu, K.-G.; Huang, H. Dependence of initial silica scaling on the surface physiochemical properties of reverse osmosis membranes during bench-scale brackish water desalination. Water Res. 2019, 150, 358–367.
- Bush, J.A.; Vanneste, J.; Gustafson, E.M.; Waechter, C.A.; Jassby, D.; Turchi, C.S.; Cath, T.Y. Prevention and management of silica scaling in membrane distillation using pH adjustment. J. Membr. Sci. 2018, 554, 366–377.
- Neofotisou, E.; Demadis, K.D. Use of antiscalants for mitigation of silica fouling and deposition: Fundamental and applications in desalination systems. Desalination 2004, 167, 257–272.
- Den, W.; Wang, C.J. Removal of silica from brackish water by electrocoagulation pretreatment to prevent fouling of reverse osmosis membranes. Sep. Purif. Technol. 2008, 59, 318–325.
- Tsai, J.-H.; Macedonio, F.; Drioli, E.; Giorno, L.; Chou, C.-Y.; Hu, F.-C.; Li, C.-L.; Chuang, C.-J.; Tung, K.-L. Membrane-based zero liquid discharge: Myth or reality? J. Taiwan Inst. Chem. Eng. 2017, 80, 192–202.
- Qiu, T.; Davies, P.A. Comparison of configurations for high-recovery inland desalination systems. Water 2012, 4, 690–706.
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23.
- Bond, R.; Warner, J. Zero Liquid Discharge; The Water Research Foundation: Alexandria, VA, USA, 2011.
- Bond, R.; Batchelor, B.; Davis, T.; Klayman, B. Zero liquid discharge desalination of brackish water with an innovative form of electrodialysis: Electrodialysis metathesis. Fla. Water Resour. J. 2011, 63, 36–44.
- Efraty, A. Closed circuit desalination series no-3: High recovery low energy desalination of brackish water by a new two-mode consecutive sequential method. Desalin. Water Treat. 2012, 42, 256–261.
- Loganathan, K.; Chelme-Ayala, P.; Gamal El-Din, M. Treatment of basal water using a hybrid electrodialysis reversal–reverse osmosis system combined with a low-temperature crystallizer for near-zero liquid discharge. Desalination 2015, 363, 92–98.
- Zhang, C.; Shi, Y.; Li, H.; Li, R.; Hong, S.; Zhuo, S.; Zhang, T.; Wang, P. Designing a next generation solar crystallizer for real seawater brine treatment with zero liquid discharge. Nat. Commun. 2021, 12, 998.
- van Wyk, S.; van der Ham, A.G.J.; Kersten, S.R.A. Potential of supercritical water desalination (SCWD) as zero liquid discharge (ZLD) technology. Desalination 2020, 495, 114593.
- Odu, S.O.; van der Ham, A.G.J.; Metz, S.; Kersten, S.R.A. Design of a process for supercritical water desalination with zero liquid discharge. Ind. Eng. Chem. Res. 2015, 54, 5527–5535.
- Son, H.S.; Soukane, S.; Lee, J.; Kim, Y.; Kim, Y.-D.; Ghaffour, N. Towards sustainable circular brine reclamation using seawater reverse osmosis, membrane distillation and forward osmosis hybrids: An experimental investigation. J. Environ. Manag. 2021, 293, 112836.
More
Information
Subjects:
Energy & Fuels
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.2K
Revisions:
2 times
(View History)
Update Date:
25 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No