| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Barouch Giechaskiel | + 1976 word(s) | 1976 | 2021-08-16 12:17:23 | | | |
| 2 | Beatrix Zheng | Meta information modification | 1976 | 2021-08-19 10:28:52 | | |
Video Upload Options
In a Fourier Transform InfraRed (FTIR) spectrometer, some of the infrared (IR) radiation is absorbed by the sample, and some of it is passed through (transmitted). The resulting molecular absorption and transmission response can be used to identify the components of the sample and their concentration.
1. Introduction
One method that can measure many compounds is FTIR (Fourier transform infrared) spectroscopy (= study of the interaction between light with matter) [1]. Many compounds absorb infrared energy at an intrinsic wave number (or wavelength) proportionally to their concentration. In an FTIR spectrometer, some of the infrared (IR) radiation is absorbed by the sample, and some of it is passed through (transmitted). The resulting molecular absorption and transmission response can be used to identify the components of the sample and their concentration. FTIR, compared to other IR techniques, can measure many components in real-time due to the use of an interferometer that allows the collection of a broad range of wavelengths. By contrast, non-dispersive infrared (NDIR) analyzers measure one compound due to the use of an optical filter that allows the selection of a narrow wavelength area, specific to the compound of interest.
1.1. FTIR Description
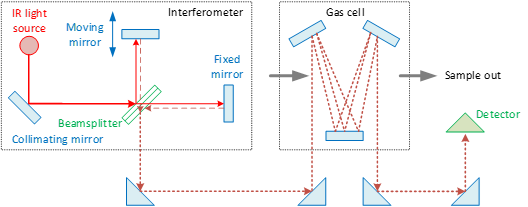
The heart of every FTIR instrument is an optical device called an interferometer (Figure 1) [1]. The oldest and most common type is the Michelson interferometer. The infrared source is usually a heated ceramic (at ca. 1200 °C). A collimating mirror collects light from the source and makes its rays parallel. A beamsplitter (in KBr) transmits approximately half of the light incident upon it and reflects the remaining half. A fraction of the light transmitted travels to a fixed mirror, while the other fraction travels to a moving mirror (see Figure 1). The lights are reflected by the two mirrors back to the beamsplitter, where they are recombined into a single light beam. This light beam interacts with the sample (exhaust gas) in a gas cell and finally strikes the detector. A multireflection cell is used to obtain a long optical path length with the minimum possible volume of the cell [2].
Because the path that one beam travels is a fixed length and the other is constantly changing as its mirror moves, the signal which exits the interferometer is the result of these two beams “interfering” with each other. The resulting signal is called an interferogram (i.e., a plot of light intensity versus optical path difference). The interferograms measured are then Fourier transformed to yield a spectrum (i.e., a plot intensity versus frequency/wavenumber). There is also a laser (not shown in the figure) whose light follows the infrared beam. This laser light is used to measure the optical path difference of the interferometer. The spectral resolution (in cm−1) depends on the inverse of the optical path difference.
Figure 1. Principle of operation of FTIR (Fourier transform infrared) spectroscopy. IR = infrared.
1.2. FTIR in the Vehicle Exhaust Regulations
Vehicle emissions are regulated since the 1970s [3]. The measurements are conducted on chassis dynamometers (light-duty vehicles) or in engine test cells (heavy-duty engines). The instruments described in the regulations are sampling from the full dilution tunnel, where the whole exhaust gas is diluted, or directly from the tailpipe (undiluted exhaust). The control of the regulated pollutants (e.g., CO, NOx ) with advanced aftertreatment devices [4] has led in some cases to increased emission of non-regulated pollutants (e.g., N2O, NH3).
The measurement techniques for regulated pollutants are well-defined in the regulation (e.g., non-dispersive infrared (NDIR) for CO and CO2). For non-regulated pollutants, only recently, a Global Technical Regulation for light-duty vehicles (GTR 15) prescribes possible measurement techniques.
GTR 15 allows the use of FTIR for ethanol, formaldehyde, acetaldehyde, and N2O only from the dilution tunnel. This is because there is no exhaust gas measurement to determine the emissions from the tailpipe. Only NH3 has to be measured from the tailpipe. At the moment, there is a limit only for heavy-duty engines (in ppm) in the EU regulation (not in GTR). Such specifications would need two FTIRs for the measurement of non-regulated pollutants (e.g., one for NH3 at the tailpipe and one at the dilution tunnel for the other pollutants). Permitting measurement of all pollutants from the tailpipe would simplify the setup. Furthermore, FTIR could be used instead of other analyzers. For example typically NDIR analyzers are used for CO and CO2, chemiluminescence detectors (CLD) for NOx, and flame ionization detectos (FID) for hydrocarbons. Indeed, the use of FTIR at the tailpipe is a commonly accepted technique for research and development.
1.3. FTIR for Vehicle Exhaust Research
FTIR spectroscopy is used in geology, chemistry, materials, medicine, and biology research fields on solid, liquid, and gaseous samples. FTIR has been used in a wide range of air pollution-related studies in both ambient air and environmental chambers. Already in the late 1970s, an FTIR system was installed in a van for air pollution measurements for the Environmental Protection Agency (EPA) of the United States of America (USA).
FTIR spectroscopy has been used for the measurement of gas concentrations for various studies, e.g., soot oxidation [5], or SCR (selective catalytic reduction for NOx ) [6][7] and catalyst evaluation [8][9][10] with synthetic gases. It has also been used in engine test beds to assess ethanol [11][12], biodiesel [13] such as Jatropha [14], dimethyl ether (DME) [15], or hydro-treated vegetable oil (HVO) [16], homogeneous charge compression ignition (HCCI) engines [17], gasoline compression ignition engine [18], post injection effect on emissions [19], NH3 sensors [20], or even modeling of emissions [21]. FTIR instruments have also been used on chassis dynamometers: Small gasoline engines [22] or even diesel trucks [23][24]. For example, for exhaust gas recirculation (EGR) [25], alternative fuels [26][27], reactive nitrogen compounds [28][29], impact of low temperature on non-regulated pollutants [30], and retrofit evaluation [31][32] of diesel vehicles. Similarly, chassis dynamometer studies with gasoline vehicles [33] focused on unregulated emissions [34][35][36][37][38], NH3 [39][40][41][42][43], effect of exhaust gas reforming on emissions [44], low temperature [30][45][46], alternative fuels [35][47], and hybrids [48][49][50]. Motorcycles’ non-regulated pollutants emissions have also been assessed with FTIR [51][52][53][54][55].
The on-road application started in 2000 [56]. Since then other researchers measured emissions on the road [57][58][59], greenhouse gases (GHG) [60], nitrogen species [61], cold start emissions [62][63][64][65][66] of gasoline vehicles and the impact of ambient temperature [67]. A few also studied compressed natural gas (CNG) [68], diesel fueled vehicles [69][70][71] and their non-regulated pollutants [72].
2. Results and Discussion
2.1. Comparison with Other Methods
2.2. FTIR and Interferences
3. Conclusions
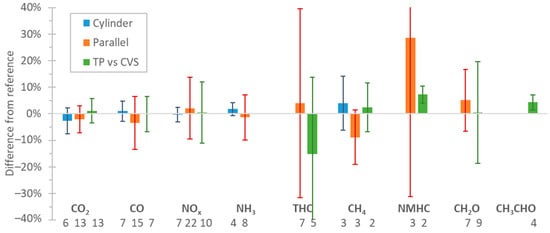
Based on studies that assessed FTIRs performance on the measurement of vehicle exhaust emissions, the mean differences compared to regulated or other methods were around ±2.5% for CO2, CO, NOx, and NH3 with a variability (one standard deviation) of 5% for CO2 and 10% for CO, NOx , and NH3. For CH4, acetaldehyde, and formaldehyde, the mean differences were ±10% (variability 10–20%), but for total hydrocarbons, much higher differences were noticed. The differences were similar regardless of the sampling location of the FTIR (dilution tunnel or tailpipe). Assessment of prototype portable FTIRs on the road confirmed these findings also on-board, but for a narrow range of environmental and driving conditions. Based on these results, FTIRs may be an alternative for on-road testing. However, more studies with commercial portable systems are necessary to cover a wider range of environmental and driving conditions. The introduction of FTIRs in the regulation will require strict technical and performance requirements and procedures based on recently developed standards.
References
- Smith, B.C. Fundamentals of Fourier Transform Infrared Spectroscopy, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-4200-6929-7.
- White, J.U. Long Optical Paths of Large Aperture. J. Opt. Soc. Am. 1942, 32, 285.
- Berg, W. Legislation for the reduction of exhaust gas emissions. In Traffic and Environment; Gruden, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 3T, pp. 175–253. ISBN 978-3-540-00050-1.
- Keenan, M. Exhaust Emissions Control: 60 Years of Innovation and Development; SAE Technical Paper 2017-24-0120: Warrendale, PA, USA, 2017.
- Soltani, S.; Andersson, R.; Andersson, B. The Effect of Exhaust Gas Composition on the Kinetics of Soot Oxidation and Diesel Particulate Filter Regeneration. Fuel 2018, 220, 453–463.
- Stanciulescu, M.; Charland, J.-P.; Kelly, J.F. Effect of Primary Amine Hydrocarbon Chain Length for the Selective Catalytic Reduction of NOx from Diesel Engine Exhaust. Fuel 2010, 89, 2292–2298.
- Zhao, W.; Liu, Y.; Wei, H.; Zhang, R.; Luo, G.; Hou, H.; Chen, S.; Zhang, R. NO Removal by Plasma-Enhanced NH3-SCR Using Methane as an Assistant Reduction Agent at Low Temperature. Appl. Sci. 2019, 9, 2751.
- Davies, C.; Thompson, K.; Cooper, A.; Golunski, S.; Taylor, S.H.; Bogarra Macias, M.; Doustdar, O.; Tsolakis, A. Simultaneous Removal of NOx and Soot Particulate from Diesel Exhaust by In-Situ Catalytic Generation and Utilisation of N2O. Appl. Catal. B Environ. 2018, 239, 10–15.
- Wang, C.; Zhang, C.; Zhao, Y.; Yan, X.; Cao, P. Poisoning Effect of SO2 on Honeycomb Cordierite-Based Mn–Ce/Al2O3 Catalysts for NO Reduction with NH3 at Low Temperature. Appl. Sci. 2018, 8, 95.
- Auvinen, P.; Nevalainen, P.; Suvanto, M.; Oliva, F.; Llamas, X.; Barciela, B.; Sippula, O.; Kinnunen, N.M. A Detailed Study on Regeneration of SO2 Poisoned Exhaust Gas after-Treatment Catalysts: In Pursuance of High Durability and Low Methane, NH3 and N2O Emissions of Heavy-Duty Vehicles. Fuel 2021, 291, 120223.
- de Melo, T.C.C.; Machado, G.B.; Belchior, C.R.; Colaço, M.J.; Barros, J.E.; de Oliveira, E.J.; de Oliveira, D.G. Hydrous Ethanol–Gasoline Blends—Combustion and Emission Investigations on a Flex-Fuel Engine. Fuel 2012, 97, 796–804.
- Gailis, M.; Pirs, V.; Jansons, M.; Birzietis, G.; Dukulis, I. An Experimental Investigation on Aldehyde and Methane Emissions from Hydrous Ethanol and Gasoline Fueled SI Engine; SAE Technical Paper 2020-01-2047: Warrendale, PA, USA, 2020.
- Liu, S.; Chen, W.; Zhu, Z.; Jiang, S.; Ren, T.; Guo, H. A Review of the Developed New Model Biodiesels and Their Effects on Engine Combustion and Emissions. Appl. Sci. 2018, 8, 2303.
- Tan, P.; Hu, Z.; Lou, D.; Li, Z. Exhaust Emissions from a Light-Duty Diesel Engine with Jatropha Biodiesel Fuel. Energy 2012, 39, 356–362.
- Yu-sheng, Z.; Chun-lan, M.; Hai-ying, S.; Shao-ren, Z. Study on Formaldehyde Emission in a DME-Fueled Direct-Injection Diesel Engine; SAE Technical Paper 2007-01-1909: Warrendale, PA, USA, 2007.
- Hunicz, J.; Matijošius, J.; Rimkus, A.; Kilikevičius, A.; Kordos, P.; Mikulski, M. Efficient Hydrotreated Vegetable Oil Combustion under Partially Premixed Conditions with Heavy Exhaust Gas Recirculation. Fuel 2020, 268, 117350.
- Lemel, M.; Hultqvist, A.; Vressner, A.; Nordgren, H.; Persson, H.; Johansson, B. Quantification of the Formaldehyde Emissions from Different HCCI Engines Running on a Range of Fuels; SAE Technical Paper 2005-01-3724: Warrendale, PA, USA, 2005.
- Jiang, C.; Huang, G.; Liu, G.; Qian, Y.; Lu, X. Optimizing Gasoline Compression Ignition Engine Performance and Emissions: Combined Effects of Exhaust Gas Recirculation and Fuel Octane Number. Appl. Therm. Eng. 2019, 153, 669–677.
- Wu, Y.; Wang, P.; Farhan, S.M.; Yi, J.; Lei, L. Effect of Post-Injection on Combustion and Exhaust Emissions in DI Diesel Engine. Fuel 2019, 258, 116131.
- Brosha, E.L.; Prikhodko, V.Y.; Kreller, C.R.; Pihl, J.A.; Curran, S.; Parks, J.E.; Mukundan, R. Response Characteristics of Stable Mixed-Potential NH3 Sensors in Diesel Engine Exhaust. Emiss. Control Sci. Technol. 2017, 3, 112–121.
- Zarante, P.H.B.; Sodré, J.R. Comparison of Aldehyde Emissions Simulation with FTIR Measurements in the Exhaust of a Spark Ignition Engine Fueled by Ethanol. Heat Mass Transf. 2018, 54, 2079–2087.
- Zardini, A.A.; Suarez-Bertoa, R.; Forni, F.; Montigny, F.; Otura-Garcia, M.; Carriero, M.; Astorga, C. Reducing the Exhaust Emissions of Unregulated Pollutants from Small Gasoline Engines with Alkylate Fuel and Low-Ash Lube Oil. Environ. Res. 2019, 170, 203–214.
- Yamada, H.; Misawa, K.; Suzuki, D.; Tanaka, K.; Matsumoto, J.; Fujii, M.; Tanaka, K. Detailed Analysis of Diesel Vehicle Exhaust Emissions: Nitrogen Oxides, Hydrocarbons and Particulate Size Distributions. Proc. Combust. Inst. 2011, 33, 2895–2902.
- Clairotte, M.; Suarez-Bertoa, R.; Zardini, A.A.; Giechaskiel, B.; Pavlovic, J.; Valverde, V.; Ciuffo, B.; Astorga, C. Exhaust Emission Factors of Greenhouse Gases (GHGs) from European Road Vehicles. Environ. Sci. Eur. 2020, 32, 125.
- Nevius, T.; Rooney, R.; Zummer, R. Speciation of Nitrogen Oxides in a Light Duty Diesel Engine during an EGR System Failure; SAE Technical Paper 2012-01-0876: Warrendale, PA, USA, 2012.
- Aakko-Saksa, P.; Koponen, P.; Roslund, P.; Laurikko, J.; Nylund, N.-O.; Karjalainen, P.; Rönkkö, T.; Timonen, H. Comprehensive Emission Characterisation of Exhaust from Alternative Fuelled Cars. Atmos. Environ. 2020, 236, 117643.
- Suarez-Bertoa, R.; Kousoulidou, M.; Clairotte, M.; Giechaskiel, B.; Nuottimäki, J.; Sarjovaara, T.; Lonza, L. Impact of HVO Blends on Modern Diesel Passenger Cars Emissions during Real World Operation. Fuel 2019, 235, 1427–1435.
- Bielaczyc, P.; Szczotka, A.; Woodburn, J. An Overview of Emissions of Reactive Nitrogen Compounds from Modern Light Duty Vehicles Featuring SI Engines. Combust. Engines 2014, 159, 48–53.
- Andersson, J.; May, J.; Favre, C.; Bosteels, D.; de Vries, S.; Heaney, M.; Keenan, M.; Mansell, J. On-Road and Chassis Dynamometer Evaluations of Emissions from Two Euro 6 Diesel Vehicles. SAE Int. J. Fuels Lubr. 2014, 7, 919–934.
- Suarez-Bertoa, R.; Astorga, C. Impact of Cold Temperature on Euro 6 Passenger Car Emissions. Environ. Pollut. 2018, 234, 318–329.
- Giechaskiel, B.; Suarez-Bertoa, R.; Lähde, T.; Clairotte, M.; Carriero, M.; Bonnel, P.; Maggiore, M. Evaluation of NOx Emissions of a Retrofitted Euro 5 Passenger Car for the Horizon Prize “Engine Retrofit”. Environ. Res. 2018, 166, 298–309.
- Giechaskiel, B.; Suarez-Bertoa, R.; Lahde, T.; Clairotte, M.; Carriero, M.; Bonnel, P.; Maggiore, M. Emissions of a Euro 6b Diesel Passenger Car Retrofitted with a Solid Ammonia Reduction System. Atmosphere 2019, 10, 180.
- Hirschberger, R. Automotive Emissions Analysis with Spectroscopic Techniques. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation, 1st ed.; Meyers, R., Ed.; Wiley: New York, NY, USA, 2006; ISBN 978-0-471-97670-7.
- Aakko-Saksa, P.; Rantanen-Kolehmainen, L.; Koponen, P.; Engman, A.; Kihlman, J. Biogasoline Options—Possibilities for Achieving High Bio-Share and Compatibility with Conventional Cars. SAE Int. J. Fuels Lubr. 2011, 4, 298–317.
- Aakko-Saksa, P.T.; Rantanen-Kolehmainen, L.; Skyttä, E. Ethanol, Isobutanol, and Biohydrocarbons as Gasoline Components in Relation to Gaseous Emissions and Particulate Matter. Environ. Sci. Technol. 2014, 48, 10489–10496.
- Suarez-Bertoa, R.; Zardini, A.A.; Keuken, H.; Astorga, C. Impact of Ethanol Containing Gasoline Blends on Emissions from a Flex-Fuel Vehicle Tested over the Worldwide Harmonized Light Duty Test Cycle (WLTC). Fuel 2015, 143, 173–182.
- Suarez-Bertoa, R.; Lähde, T.; Pavlovic, J.; Valverde, V.; Clairotte, M.; Giechaskiel, B. Laboratory and On-Road Evaluation of a GPF-Equipped Gasoline Vehicle. Catalysts 2019, 9, 678.
- Mehsein, K.; Norsic, C.; Chaillou, C.; Nicolle, A. Minimizing Secondary Pollutant Formation through Identification of Most Influential Volatile Emissions in Gasoline Exhausts: Impact of the Vehicle Powertrain Technology. Atmos. Environ. 2020, 226, 117394.
- Durbin, T.D.; Wilson, R.D.; Norbeck, J.M.; Miller, J.W.; Huai, T.; Rhee, S.H. Estimates of the Emission Rates of Ammonia from Light-Duty Vehicles Using Standard Chassis Dynamometer Test Cycles. Atmos. Environ. 2002, 36, 1475–1482.
- Woodburn, J.; Bielaczyc, P.; Szczotka, A. Chassis Dynamometer Testing of Ammonia Emissions from Light-Duty Si Vehicles in the Context of Emissions of Reactive Nitrogen Compounds; SAE Technical Paper 2013-01-1346: Warrendale, PA, USA, 2013.
- Suarez-Bertoa, R.; Zardini, A.A.; Astorga, C. Ammonia Exhaust Emissions from Spark Ignition Vehicles over the New European Driving Cycle. Atmos. Environ. 2014, 97, 43–53.
- Wang, X.; Ge, Y.; Gong, H.; Yang, Z.; Tan, J.; Hao, L.; Su, S. Ammonia Emissions from China-6 Compliant Gasoline Vehicles Tested over the WLTC. Atmos. Environ. 2019, 199, 136–142.
- Liu, Y.; Wang, H.; Li, N.; Tan, J.; Chen, D. Research on Ammonia Emissions from Three-Way Catalytic Converters Based on Small Sample Test and Vehicle Test. Sci. Total Environ. 2021, 795, 148926.
- Bogarra, M.; Herreros, J.M.; Tsolakis, A.; York, A.P.E.; Millington, P.J. Study of Particulate Matter and Gaseous Emissions in Gasoline Direct Injection Engine Using On-Board Exhaust Gas Fuel Reforming. Appl. Energy 2016, 180, 245–255.
- Clairotte, M.; Adam, T.W.; Zardini, A.A.; Manfredi, U.; Martini, G.; Krasenbrink, A.; Vicet, A.; Tournié, E.; Astorga, C. Effects of Low Temperature on the Cold Start Gaseous Emissions from Light Duty Vehicles Fuelled by Ethanol-Blended Gasoline. Appl. Energy 2013, 102, 44–54.
- Suarez-Bertoa, R.; Astorga, C. Isocyanic Acid and Ammonia in Vehicle Emissions. Transp. Res. Part D Transp. Environ. 2016, 49, 259–270.
- Huai, T.; Durbin, T.; Rhee, S.H.; Norbeck, J.M. Investigation of Emission Rates of Ammonia, Nitrous Oxide and Other Exhaust Compounds from Alternative Fuel Vehicles Using a Chassis Dynamometer. Int. J. Automot. Technol. 2003, 4, 9–19.
- Reyes, F.; Grutter, M.; Jazcilevich, A.; González-Oropeza, R. Tecnical Note: Analysis of Non-Regulated Vehicular Emissions by Extractive FTIR Spectrometry: Tests on a Hybrid Car in Mexico City. Atmos. Chem. Phys. 2006, 6, 5339–5346.
- Suarez-Bertoa, R.; Astorga, C. Unregulated Emissions from Light-Duty Hybrid Electric Vehicles. Atmos. Environ. 2016, 136, 134–143.
- Suarez-Bertoa, R.; Pavlovic, J.; Trentadue, G.; Otura-Garcia, M.; Tansini, A.; Ciuffo, B.; Astorga, C. Effect of Low Ambient Temperature on Emissions and Electric Range of Plug-in Hybrid Electric Vehicles. ACS Omega 2019, 4, 3159–3168.
- Daemme, L.C.; Penteado, R.d.A.; Furlan, C.; Errera, M.; Zotin, F.M.Z. An Investigation on Aldehyde and Ammonia Emissions from a 4-Stroke Gasoline-Fueled Motorcycle. Ammonia Emission Reduction by Using a SCR Catalyst; SAE Technical Paper 2013-36-0181: Warrendale, PA, USA, 2013.
- Daemme, L.C.; Penteado, R.; Zotin, F.; Errera, M. Regulated and Unregulated Emissions from a Flex Fuel Motorcycle Fuelled with Various Gasoline/Ethanol Blends; SAE Technical Paper 2014-32-0032: Warrendale, PA, USA, 2014.
- Daemme, L.C.; Penteado, R.; Zotin, F.; Corrêa, S.M.; Errera, M.R.; Forcetto, A. The Effect of Fuel Sulfur Content on Ammonia, Aldehyde and Regulated Emissions Emitted from a Euro Iii Motorcycle; SAE Technical Paper 2016-36-0158: Warrendale, PA, USA, 2016.
- Clairotte, M.; Adam, T.W.; Chirico, R.; Giechaskiel, B.; Manfredi, U.; Elsasser, M.; Sklorz, M.; DeCarlo, P.F.; Heringa, M.F.; Zimmermann, R.; et al. Online Characterization of Regulated and Unregulated Gaseous and Particulate Exhaust Emissions from Two-Stroke Mopeds: A Chemometric Approach. Anal. Chim. Acta 2012, 717, 28–38.
- Zardini, A.A.; Platt, S.M.; Clairotte, M.; El Haddad, I.; Temime-Roussel, B.; Marchand, N.; Ježek, I.; Drinovec, L.; Močnik, G.; Slowik, J.G.; et al. Effects of Alkylate Fuel on Exhaust Emissions and Secondary Aerosol Formation of a 2-Stroke and a 4-Stroke Scooter. Atmos. Environ. 2014, 94, 307–315.
- Jetter, J.; Maeshiro, S.; Hatcho, S.; Klebba, R. Development of an On-Board Analyzer for Use on Advanced Low Emission Vehicles; SAE Technical Paper 2000-01-1140: Warrendale, PA, USA, 2000.
- Li, H.; Andrews, G.E.; Khan, A.A.; Savvidis, D.; Daham, B.; Bell, M.; Tate, J.; Ropkins, K. Analysis of Driving Parameters and Emissions for Real World Urban Driving Cycles Using an On-Board Measurement Method for a Euro 2 SI Car; SAE Technical Paper 2007-01-2066: Warrendale, PA, USA, 2007.
- Li, H.; Andrews, G.E.; Savvidis, D.; Daham, B.; Ropkins, K.; Bell, M.; Tate, J. Comparisons of the Exhaust Emissions for Different Generations of SI Cars under Real World Urban Driving Conditions; SAE Technical Paper 2008-01-0754: Warrendale, PA, USA, 2008.
- Khalfan, A.; Andrews, G.; Li, H. Real World Driving: Emissions in Highly Congested Traffic; SAE Technical Paper 2017-01-2388: Warrendale, PA, USA, 2017.
- Li, H.; Khalfan, A.; Andrews, G. Determination of GHG Emissions, Fuel Consumption and Thermal Efficiency for Real World Urban Driving Using a SI Probe Car. SAE Int. J. Engines 2014, 7, 1370–1381.
- Khalfan, A.; Li, H.; Andrews, G. Speciation of Nitrogen Compounds in the Tailpipe Emissions from a SI Car under Real World Driving Conditions. SAE Int. J. Engines 2014, 7, 1961–1983.
- Collins, J.F.; Shepherd, P.; Durbin, T.D.; Lents, J.; Norbeck, J.; Barth, M. Measurements of In-Use Emissions from Modern Vehicles Using an on-Board Measurement System. Environ. Sci. Technol. 2007, 41, 6554–6561.
- Li, H.; Andrews, G.E.; Savvidis, D.; Daham, B.; Ropkins, K.; Bell, M.; Tate, J.E. Characterization of Regulated and Unregulated Cold Start Emissions for Different Real World Urban Driving Cycles Using a SI Passenger Car; SAE Technical Paper 2008-01-1648: Warrendale, PA, USA, 2008.
- Li, H.; Andrews, G.E.; Savvidis, D.; Ropkins, K.; Tate, J.; Bell, M. Investigation of Regulated and Non-Regulated Cold Start Emissions Using a Euro 3 SI Car as a Probe Vehicle under Real World Urban Driving Conditions; SAE Technical Paper 2008-01-2428: Warrendale, PA, USA, 2008.
- Sentoff, K.M.; Robinson, M.K.; Holmén, B.A. Second-by-Second Characterization of Cold-Start Gas-Phase and Air Toxic Emissions from a Light-Duty Vehicle. Transp. Res. Rec. 2010, 2158, 95–104.
- Khalfan, A.; Li, H.; Andrews, G. Cold Start SI Passenger Car Emissions from Real World Urban Congested Traffic; SAE Technical Paper 2015-01-1064: Warrendale, PA, USA, 2015.
- Li, H.; Andrews, G.E.; Savvidis, D. Influence of Cold Start and Ambient Temperatures on Greenhouse Gas (GHG) Emissions, Global Warming Potential (GWP) and Fuel Economy for SI Car Real World Driving. SAE Int. J. Fuels Lubr. 2010, 3, 133–148.
- Vojtíšek-Lom, M.; Beránek, V.; Klír, V.; Jindra, P.; Pechout, M.; Voříšek, T. On-Road and Laboratory Emissions of NO, NO2, NH3, N2O and CH4 from Late-Model EU Light Utility Vehicles: Comparison of Diesel and CNG. Sci. Total Environ. 2018, 616–617, 774–784.
- Hadavi, S.A.; Li, H.; Przybyla, G.; Jarrett, R.; Andrews, G. Comparison of Gaseous Emissions for B100 and Diesel Fuels for Real World Urban and Extra Urban Driving. SAE Int. J. Fuels Lubr. 2012, 5, 1132–1154.
- Hadavi, S.; Andrews, G.E.; Li, H.; Przybyla, G.; Vazirian, M. Diesel Cold Start into Congested Real World Traffic: Comparison of Diesel and B100 for Ozone Forming Potential; SAE Technical Paper 2013-01-1145: Warrendale, PA, USA, 2013.
- Przybyla, G.; Hadavi, S.; Li, H.; Andrews, G.E. Real World Diesel Engine Greenhouse Gas Emissions for Diesel Fuel and B100; SAE Technical Paper 2013-01-1514: Warrendale, PA, USA, 2013.
- Suarez-Bertoa, R.; Pechout, M.; Vojtíšek, M.; Astorga, C. Regulated and Non-Regulated Emissions from Euro 6 Diesel, Gasoline and CNG Vehicles under Real-World Driving Conditions. Atmosphere 2020, 11, 204.
- Hoard, J.; Snow, R.; Xu, L.; Gierczak, C.; Hammerle, R.; Montreuil, C.; Farooq, S.I. NOx Measurement Errors in Ammonia-Containing Exhaust; SAE Technical Paper 2007-01-0330: Warrendale, PA, USA, 2007.
- Gierczak, C.A.; Kralik, L.L.; Mauti, A.; Harwell, A.L.; Maricq, M.M. Measuring NMHC and NMOG Emissions from Motor Vehicles via FTIR Spectroscopy. Atmos. Environ. 2017, 150, 425–433.
- Lamas, J.E.; Hara, K.; Mori, Y. Optimization of Automotive Exhaust Sampling Parameters for Evaluation of after-Treatment Systems Using FTIR Exhaust Gas Analyzers; SAE Technical Paper 2019-01-0746: Warrendale, PA, USA, 2019.
- Woodburn, J. Emissions of Reactive Nitrogen Compounds (RNCs) from Two Vehicles with Turbo-Charged Spark Ignition Engines over Cold Start Driving Cycles. Combust. Engines 2021.
- Hoard, J.; Venkataramanan, N.; Marshik, B.; Murphy, W. NH3 Storage in Sample Lines; SAE Technical Paper 2014-01-1586: Warrendale, PA, USA, 2014.