
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Breygina | + 1998 word(s) | 1998 | 2021-07-16 09:38:13 | | | |
| 2 | Vicky Zhou | Meta information modification | 1998 | 2021-07-20 09:46:22 | | |
Video Upload Options
Gymnosperms are amazing representatives of the flora. On the one hand, they are ancient plants with primitive characteristics of anatomical structure; on the other hand, they are perfectly adapted to their habitat and are the dominant species in many ecosystems due to their impressive size and longevity, with their reproductive system being of particular interest. It has progressive features, because in this group the reduced male gametophyte—pollen grain—first appeared, as well as the ability to form seeds. In addition, this group still represents a wide variety of reproductive patterns, strategies, and relationships. For example, the degree of gametophyte reduction varies, there are both zooidogamy and siphonogamy, and the reproductive process can both be relatively fast and last over several years.
1. Introduction
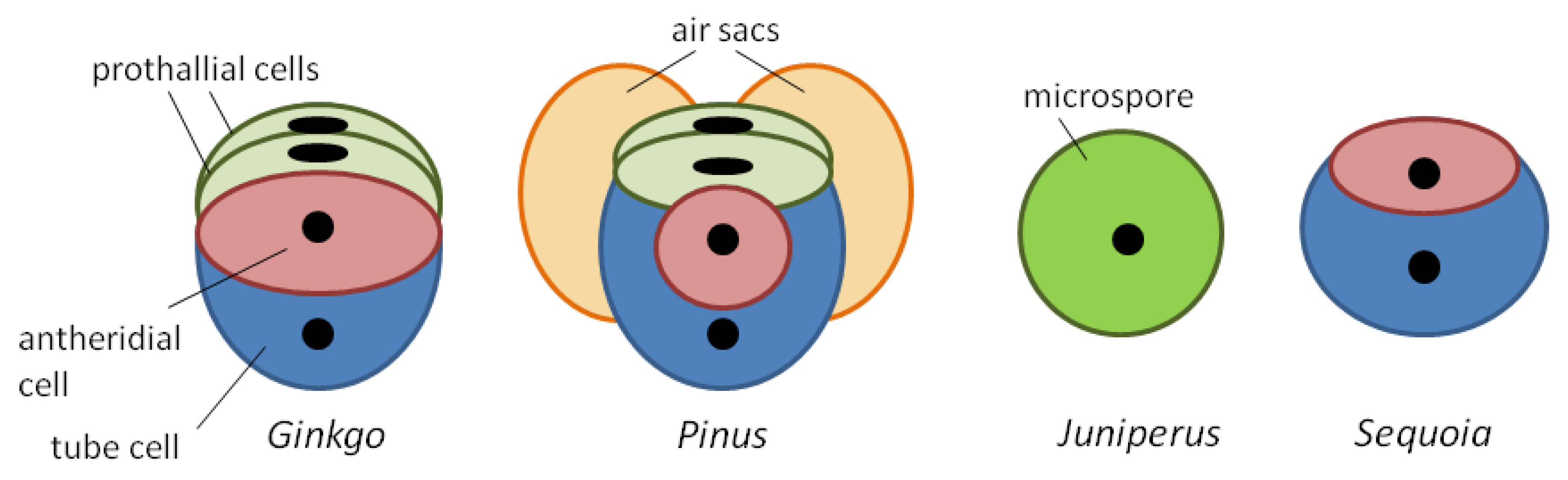 Figure 1. Cellular composition of some gymnosperm pollen grains at dehiscence.
Figure 1. Cellular composition of some gymnosperm pollen grains at dehiscence.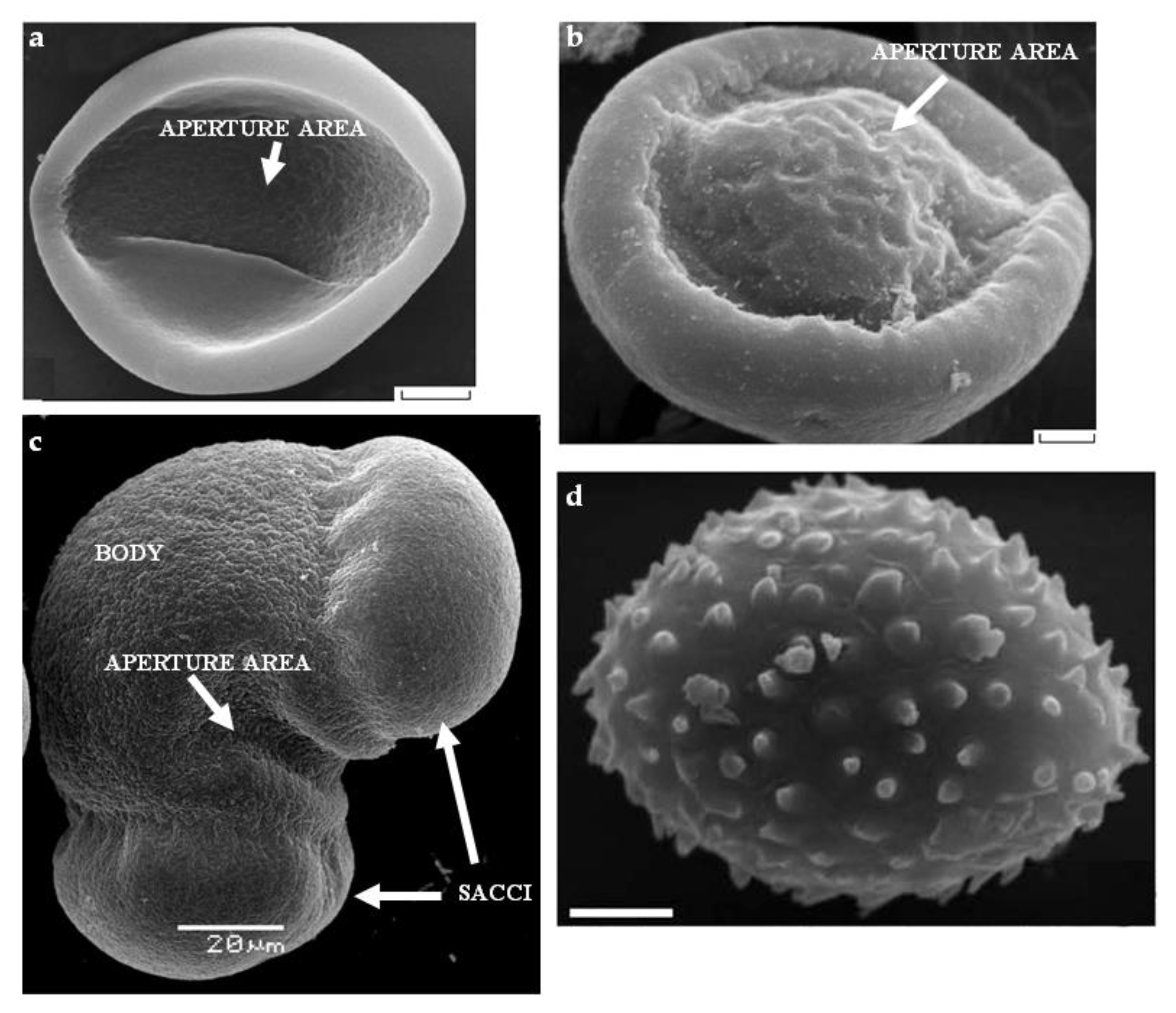 Figure 2. SEM of gymnosperm pollen: (a) Cycas micholitzii Dyer (Cycadaceae); (b) Ginkgo biloba L. with a bulge in the aperture area; (c) Picea pungens Engelm. with typical saccate morphology; (d) Gnetum macrostachyum Hook with microechinate sculpture. Scale bar: (a,b,d)—3 µm. Pictures are from the following articles: (a,b) [5], (d) [6].
Figure 2. SEM of gymnosperm pollen: (a) Cycas micholitzii Dyer (Cycadaceae); (b) Ginkgo biloba L. with a bulge in the aperture area; (c) Picea pungens Engelm. with typical saccate morphology; (d) Gnetum macrostachyum Hook with microechinate sculpture. Scale bar: (a,b,d)—3 µm. Pictures are from the following articles: (a,b) [5], (d) [6].2. Pollination and Pollen Germination in Gymnosperm Species
3. Future Perspectives
References
- Lora, J.; Hormaza, J.I.; Herrero, M. The Diversity of the Pollen Tube Pathway in Plants: Toward an Increasing Control by the Sporophyte. Front. Plant Sci. 2016, 7, 107.
- Pacini, E.; Franchi, G.G.; Ripaccioli, M. Ripe pollen structure and histochemistry of some gymnosperms. Plant Syst. Evol. 1999, 217, 81–99.
- Friedman, W.E. Growth and Development of the Male Gametophyte of Ginkgo biloba within the Ovule (in vivo). Am. J. Bot. 1987, 74, 1797–1815.
- Williams, C.G. Conifer Reproductive Biology; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-1-4020-9601-3.
- Tekleva, M.V.; Polevova, S.V.; Zavialova, N.E. On some peculiarities of sporoderm structure in members of the Cycadales and Ginkgoales. Paleontol. J. 2007, 41, 1162–1178.
- Tekleva, M.V.; Krassilov, V.A. Comparative pollen morphology and ultrastructure of modern and fossil gnetophytes. Rev. Palaeobot. Palynol. 2009, 156, 130–138.
- Halbritter, H.; Ulrich, S.; Grímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Buchner, R.; Svojtka, M.; Frosch-Radivo, A. Pollen Morphology and Ultrastructure. In Illustrated Pollen Terminology; Springer: Dordrecht, The Netherlands, 2018; pp. 37–65. ISBN 9783319713656.
- Blackmore, S.; Crane, P.R. The evolution of apertures in the spores and pollen grains of embryophytes. In Reproductive Biology in Systematics, Conservation and Economic Botany; Owens, S.J., Rudall, P.J., Eds.; Royal Botanic Gardens Kew: Richmond, UK, 1998; pp. 159–182. ISBN 1900347628.
- Tulecke, W. The Pollen of Ginkgo biloba: In Vitro Culture and Tissue Formation. Am. J. Bot. 1957, 44, 602–608.
- Lu, Y.; Wang, L.; Wang, D.; Wang, Y.; Zhang, M.; Jin, B.; Chen, P. Male cone morphogenesis, pollen development and pollen dispersal mechanism in Ginkgo biloba L. Can. J. Plant Sci. 2011, 91, 971–981.
- Lu, Y.; Zhang, L.; Cheng, F.; Zhao, J.; Cui, J.; Li, W.; Wang, L.; Jin, B. The morphology, ultrastructure, element distribution and motion behaviour in pollen of Ginkgo biloba L. Trees 2016, 30, 2189–2201.
- Dehgan, B.; Dehgan, N.B. Comparative pollen morphology and taxonomic affinities in cycadales. Am. J. Bot. 1988, 75, 1501–1516.
- Bolinder, K.; Niklas, K.J.; Rydin, C. Aerodynamics and pollen ultrastructure in Ephedra. Am. J. Bot. 2015, 102, 457–470.
- Rydin, C.; Hoorn, C. The Gnetales: Past and present. Grana 2016, 55, 1–4.
- Gelbart, G.; von Aderkas, P. Ovular secretions as part of pollination mechanisms in conifers. Ann. For. Sci. 2002, 59, 345–357.
- Coulter, A.; Poulis, B.A.D.; von Aderkas, P. Pollination drops as dynamic apoplastic secretions. Flora Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 482–490.
- Leslie, A.B. Flotation preferentially selects saccate pollen during conifer pollination. New Phytol. 2010, 188, 273–279.
- Lu, Y.; Jin, B.; Wang, L.; Wang, Y.; Wang, D.; Jiang, X.X.; Chen, P. Adaptation of male reproductive structures to wind pollination in gymnosperms: Cones and pollen grains. Can. J. Plant Sci. 2011, 91, 897–906.
- Franchi, G.G.; Piotto, B.; Nepi, M.; Baskin, C.C.; Baskin, J.M.; Pacini, E. Pollen and seed desiccation tolerance in relation to degree of developmental arrest, dispersal, and survival. J. Exp. Bot. 2011, 62, 5267–5281.
- Labandeira, C.C.; Kvaček, J.; Mostovski, M.B. Pollination drops, pollen, and insect pollination of Mesozoic gymnosperms. Taxon 2007, 56, 663–695.
- Klavins, S.D.; Kellogg, D.W.; Krings, M.; Taylor, E.L.; Taylor, T.N. Coprolites in a Middle Triassic cycad pollen cone: Evidence for insect pollination in early cycads? Evol. Ecol. Res. 2005, 7, 479–488.
- Nygaard, P. Studies on the Germination of Pine Pollen (Pinus mugo) in vitro. I. Growth Conditions and Effects of pH and Temperature on Germination, Tube Growth and Respiration. Physiol. Plant. 1969, 22, 338–346.
- Dawkins, M.D.; Owens, J.N. In vitro and in vivo pollen hydration, germination, and pollen-tube growth in white spruce, Picea glauca (Moench) Voss. Int. J. Plant Sci. 1993, 154, 506–521.
- Muren, R.C.; Ching, T.M.; Ching, K.I.M.K. Metabolic Study of Douglas-fir Pollen Germination in vitro. Physiol. Plant. 1979, 46, 287–292.
- Fernando, D.D.; Lazzaro, M.D.; Owens, J.N. Growth and development of conifer pollen tubes. Sex. Plant Reprod. 2005, 18, 149–162.
- Furness, C.A.; Rudall, P.J. Pollen aperture evolution—A crucial factor for eudicot success? Trends Plant Sci. 2004, 9, 154–158.
- Albert, B.; Ressayre, A.; Dillmann, C.; Carlson, A.L.; Swanson, R.J.; Gouyon, P.-H.; Dobritsa, A.A. Effect of aperture number on pollen germination, survival and reproductive success in Arabidopsis thaliana. Ann. Bot. 2018, 121, 733–740.
- Breygina, M.; Maksimov, N.; Polevova, S.; Evmenyeva, A. Bipolar pollen germination in blue spruce (Picea pungens). Protoplasma 2019, 256, 941–949.
- El-Ghazaly, G.; Rowley, J.; Hesse, M. Polarity, aperture condition and germination in pollen grains of Ephedra (Gnetales). Plant Syst. Evol. 1998, 213, 217–231.
- Surso, M. Pollination and pollen germination in common juniper (Juniperus communis: Cupressaceae). Arct. Environ. Res. 2018, 18, 162–174.
- Duhoux, E. Mechanism of exine rupture in hydrated ta×oid type of pollen. Grana 1982, 21, 1–7.
- Rydin, C.; Friis, E.M. Pollen germination in Welwitschia mirabilis Hook. f.: Differences between the polyplicate pollen producing genera of the Gnetales. Grana 2005, 44, 137–141.
- Prior, N.; Little, S.A.; Boyes, I.; Griffith, P.; Husby, C.; Pirone-Davies, C.; Stevenson, D.W.; Tomlinson, P.B.; von Aderkas, P. Complex reproductive secretions occur in all extant gymnosperm lineages: A proteomic survey of gymnosperm pollination drops. Plant Reprod. 2019, 32, 153–166.
- Little, S.; Prior, N.; Pirone, C.; von Aderkas, P. Pollen-ovule Interactions in Gymnosperms. In Reproductive Biology of Plants; CRC Press: Boca Raton, FL, USA, 2014; pp. 97–117.
- Prior, N.; Little, S.A.; Pirone, C.; Gill, J.E.; Smith, D.; Han, J.; Hardie, D.; O’Leary, S.J.B.; Wagner, R.E.; Cross, T. Application of proteomics to the study of pollination drops. Appl. Plant Sci. 2013, 1, 1300008.
- Losada, J.M.; Leslie, A.B. Why are the seed cones of conifers so diverse at pollination? Ann. Bot. 2018, 121, 1319–1331.
- Möller, M.; Mill, R.R.; Glidewell, S.M.; Masson, D.; Williamson, B.; Bateman, R.M. Comparative biology of the pollination mechanisms in Acmopyle pancheri and Phyllocladus hypophyllus (Podocarpaceae s. l.). Ann. Bot. 2000, 86, 149–158.
- Williams, J.H. Pollen Tube Growth Rates and the Diversification of Flowering Plant Reproductive Cycles. Int. J. Plant Sci. 2012, 173, 649–661.




