Gymnosperms are amazing representatives of the flora. On the one hand, they are ancient plants with primitive characteristics of anatomical structure; on the other hand, they are perfectly adapted to their habitat and are the dominant species in many ecosystems due to their impressive size and longevity, with their reproductive system being of particular interest. It has progressive features, because in this group the reduced male gametophyte—pollen grain—first appeared, as well as the ability to form seeds. In addition, this group still represents a wide variety of reproductive patterns, strategies, and relationships. For example, the degree of gametophyte reduction varies, there are both zooidogamy and siphonogamy, and the reproductive process can both be relatively fast and last over several years.
- plant reproduction
- pollen germination
- pollen tube growth
- gymnosperms
- conifers
- male gametophyte
- polar growth
1. Introduction
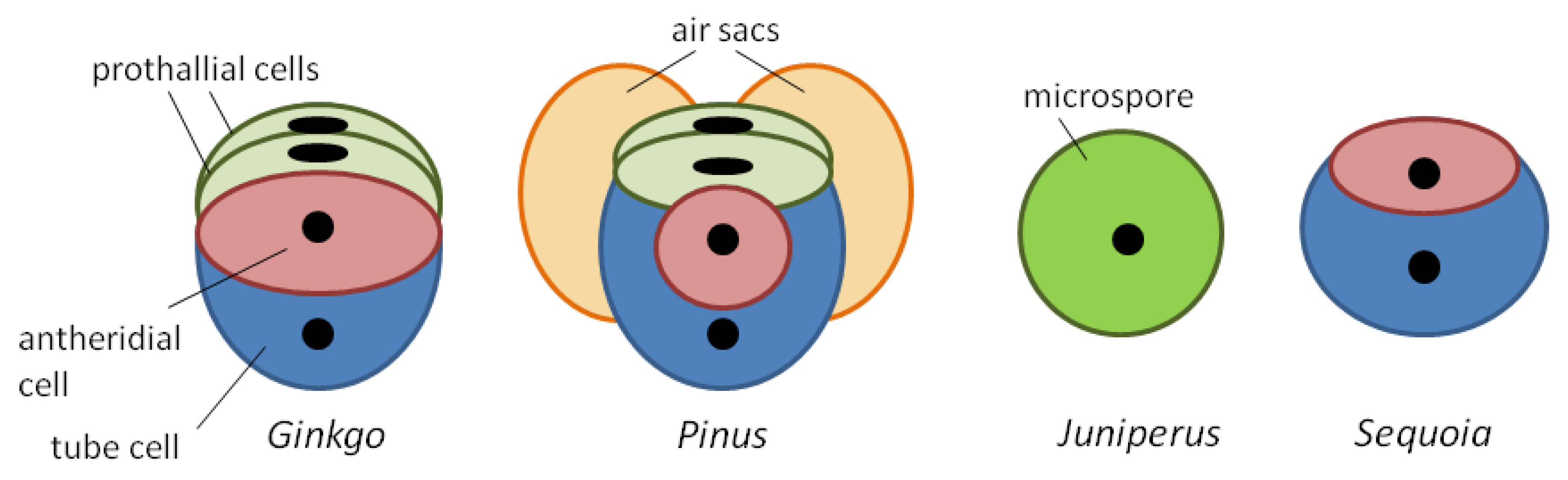 Figure 1. Cellular composition of some gymnosperm pollen grains at dehiscence.
Figure 1. Cellular composition of some gymnosperm pollen grains at dehiscence.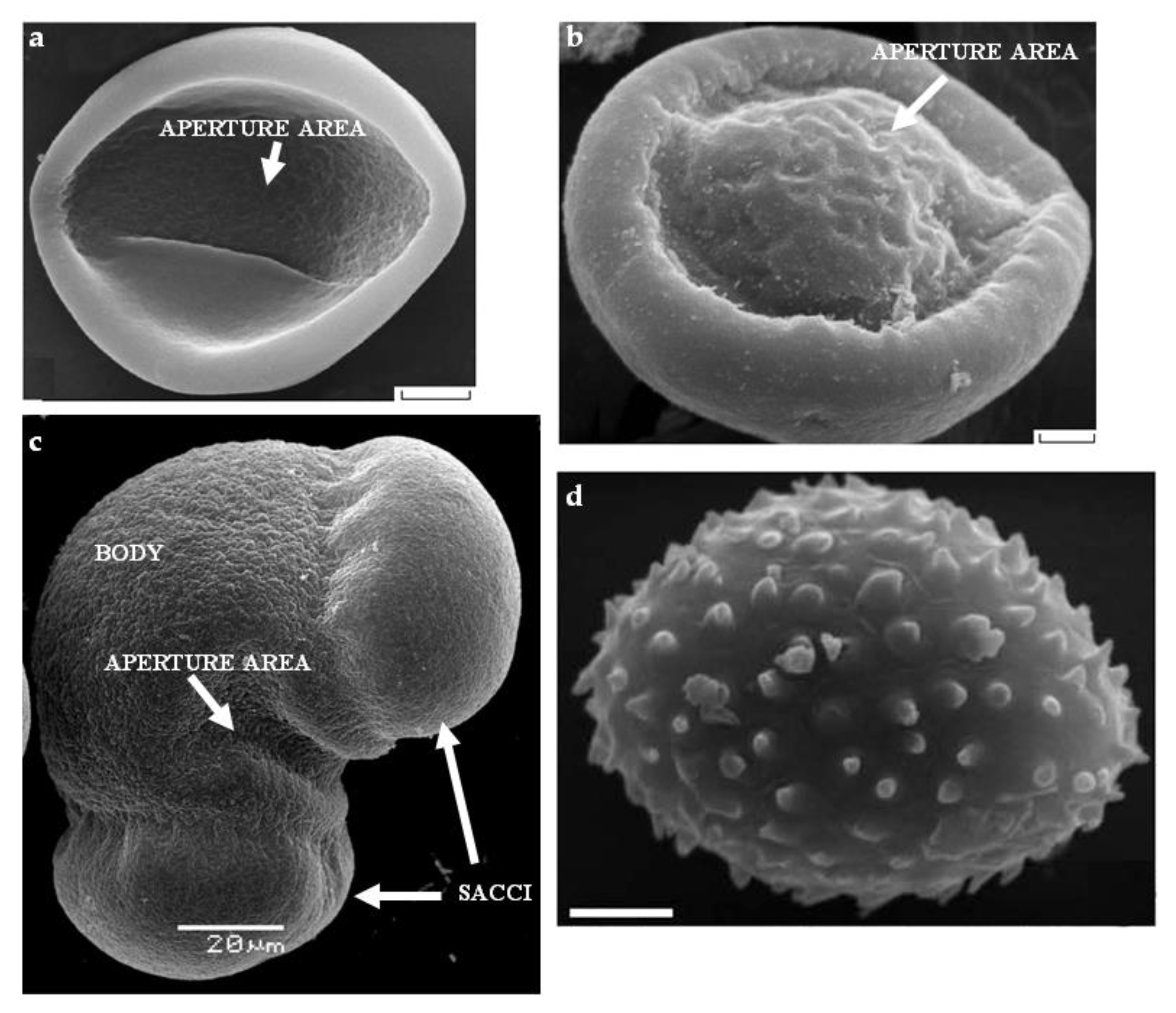 Figure 2. SEM of gymnosperm pollen: (a) Cycas micholitzii Dyer (Cycadaceae); (b) Ginkgo biloba L. with a bulge in the aperture area; (c) Picea pungens Engelm. with typical saccate morphology; (d) Gnetum macrostachyum Hook with microechinate sculpture. Scale bar: (a,b,d)—3 µm. Pictures are from the following articles: (a,b) [5], (d) [6].
Figure 2. SEM of gymnosperm pollen: (a) Cycas micholitzii Dyer (Cycadaceae); (b) Ginkgo biloba L. with a bulge in the aperture area; (c) Picea pungens Engelm. with typical saccate morphology; (d) Gnetum macrostachyum Hook with microechinate sculpture. Scale bar: (a,b,d)—3 µm. Pictures are from the following articles: (a,b) [5], (d) [6].2. Pollination and Pollen Germination in Gymnosperm Species
Approximately 98% of gymnosperm plant species are wind-pollinated [18], which largely determines their structural features (for example, specific shape of pollen grains and air sacs) and physiology. It is known that pollen of almost all modern gymnosperms, with the exception of some species of Araucariaceae and Gnetum, is characterized by a high degree of dehydration [19] and can cover great distances. Pollination with insects (mainly beetles) has been described in some Cycadales and Gnetales species [13][20], including fossils, which became possible due to the discovery of coprolites in the cones of ancient cycads [21]. Extant pollen grains have significant differences in wall structure, depending on whether they are carried by the wind or by insects, even within the same genus, which was shown for Ephedra [13]. When cultivated in vitro, pollen of many coniferous plants germinates during 1–2 days. Protocols have been developed for many species [22][23][24], allowing efficient pollen germination and monitoring of its behavior for a long time. The studies of conifer pollen tubes in vitro revealed their differences from the tubes of angiosperms, including cytoskeleton organization, regulation of organelle movement, and endo/exocytosis [25], which we will discuss in the next section. The place on the grain surface where the pollen tube appears in gymnosperms is not predetermined to the same extent as in most flowering plants. The latter in most cases have one of several apertures, intended for the fast tube outlet [26]. According to data obtained on Arabidopsis, the optimal number of apertures is three [27]. In saccate conifer pollen grains, the pollen tube emerges between the sacs, at the pole opposite to prothallial cells. Aperture area in this case is a furrow with relatively thin exine, and in its distal areas the appearance of exine ruptures is most likely [28]. However, since there are two such areas, some of the pollen grains produce not one but two tubes, which could be considered as a feature that reduces the germination rate, or as a potential adaptation. In a study conducted on pollen grains of blue spruce germinating in vitro, this phenomenon was described as “bipolar germination”, and it was found to occur only in case of optimal germination medium, and, apparently, it is not an adaptation for obtaining additional nutrients [28]. If there are no air sacs in the pollen grain, but it is polarized, then the place where the tube exits is a rather wide zone opposite to prothallial cells (Ephedra or Welwitschia pollen) [29]. No signs of polarity were found in unicellular pollen (juniper), and it is believed that the place of tube appearance is not determined [2][30][31]. In most gymnosperms, a pollen grain upon reaching the female cone lands on a pollination drop. Droplet volume ranges from 10 nL to 1 μL [33]; large drops are visible to the naked eye [13]. The list of species that do not have a pollination drop reduces as pollination is being studied closely, including through phylogenetic mapping [34]. A small or rapidly disappearing drop is often found. A pollination drop is, first of all, an apoplastic liquid. It contains inorganic substances, carbohydrates, and proteins, including enzymes [16][30][33][35]. Comparison of the pollination drop with the more studied apoplastic fluids of angiosperms (stigmatic and ovular exudates, nectar) revealed a significant similarity in their composition [16]. On this basis, it was suggested that the droplet functions are not limited to delivering pollen to the ovule [16]. Droplet enzymes, in particular chitinases, may be involved in protecting the ovule from pathogens. Possible regulatory functions of droplet components during pollen germination are also discussed. However, all these assumptions have not yet been verified experimentally. Different pollination strategies are found in conifers [36][37]. Saccate pollen of Pinus and Picea floats on the surface of the pollination drop and with it is transferred to the nucellus, where it germinates. In Abies, an analogue of a pollination drop is formed from moisture collected after rain or dew. Pollen often germinates in the micropilar canal and the tube grows towards the nucellus. In Larix, the pollination drop is absent, but the micropilar canal is filled with ovular secretion. Surrounded by this secretion, pollen hydrates, swells, and sheds exine. In this form, it floats to the nucellus, where forms a pollen tube. This usually happens a few weeks after pollination. In some Tsuga species and all Araucariaceae species, there is no pollination drop [34]—pollen lands on the conical surface next to the ovule and may remain there for several weeks. It then germinates and forms a long tube that grows through the micropyle into the ovule and reaches the nucellus. The variety of patterns of pollen behavior in the female cone is extensively discussed in the literature, since it can provide important information about the evolution of pollination [36][16][18]. Сomparing angiosperms and gymnosperms, we find that the male gametophyte of the latter often has to cover a shorter distance after pollination, but it does it for a much longer time. Male gametophyte germination and growth occur slowly at all stages: the hydration of conifer pollen usually occurs in the first day after pollination, and pollen tube appears within a few days, while in flowering plants these processes take minutes and hours [25][38]. Thus, growth rate of Picea abies pollen tube is about 20 µm/h, which is a striking contrast compared to 300–1500 µm/h in angiosperms. In addition, conifers are characterized by a period of long dormancy when the pollen tube does not grow. In particular, the tube growth can stop for the time required to complete female gametophyte development—up to a year. In this case, a few days before fertilization, the pollen tube resumes its growth and delivers sperms to the ovule. For example, in pine and some Araucariaceae, pollen germinates shortly after pollination and the tube enters the nucellus. The dormant period lasts from mid-summer to the next spring. In Pinus taeda, the dormant period of the tube starts when meiosis begins in the ovule and ends a few days before fertilization [4]. Thus, for different plant groups, the time from pollination to fertilization varies from several weeks (most species of Cupressaceae and Pinaceae) to a year (Pinus and some Araucariaceae) [4]. The review of physiological data obtained for pollen germination in gymnosperm species can be found in the review article on which this topic is based.3. Future Perspectives
References
- Lora, J.; Hormaza, J.I.; Herrero, M. The Diversity of the Pollen Tube Pathway in Plants: Toward an Increasing Control by the Sporophyte. Front. Plant Sci. 2016, 7, 107.
- Pacini, E.; Franchi, G.G.; Ripaccioli, M. Ripe pollen structure and histochemistry of some gymnosperms. Plant Syst. Evol. 1999, 217, 81–99.
- Friedman, W.E. Growth and Development of the Male Gametophyte of Ginkgo biloba within the Ovule (in vivo). Am. J. Bot. 1987, 74, 1797–1815.
- Williams, C.G. Conifer Reproductive Biology; Springer: Dordrecht, The Netherlands, 2009; ISBN 978-1-4020-9601-3.
- Tekleva, M.V.; Polevova, S.V.; Zavialova, N.E. On some peculiarities of sporoderm structure in members of the Cycadales and Ginkgoales. Paleontol. J. 2007, 41, 1162–1178.
- Tekleva, M.V.; Krassilov, V.A. Comparative pollen morphology and ultrastructure of modern and fossil gnetophytes. Rev. Palaeobot. Palynol. 2009, 156, 130–138.
- Halbritter, H.; Ulrich, S.; Grímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Buchner, R.; Svojtka, M.; Frosch-Radivo, A. Pollen Morphology and Ultrastructure. In Illustrated Pollen Terminology; Springer: Dordrecht, The Netherlands, 2018; pp. 37–65. ISBN 9783319713656.
- Blackmore, S.; Crane, P.R. The evolution of apertures in the spores and pollen grains of embryophytes. In Reproductive Biology in Systematics, Conservation and Economic Botany; Owens, S.J., Rudall, P.J., Eds.; Royal Botanic Gardens Kew: Richmond, UK, 1998; pp. 159–182. ISBN 1900347628.
- Tulecke, W. The Pollen of Ginkgo biloba: In Vitro Culture and Tissue Formation. Am. J. Bot. 1957, 44, 602–608.
- Lu, Y.; Wang, L.; Wang, D.; Wang, Y.; Zhang, M.; Jin, B.; Chen, P. Male cone morphogenesis, pollen development and pollen dispersal mechanism in Ginkgo biloba L. Can. J. Plant Sci. 2011, 91, 971–981.
- Lu, Y.; Zhang, L.; Cheng, F.; Zhao, J.; Cui, J.; Li, W.; Wang, L.; Jin, B. The morphology, ultrastructure, element distribution and motion behaviour in pollen of Ginkgo biloba L. Trees 2016, 30, 2189–2201.
- Dehgan, B.; Dehgan, N.B. Comparative pollen morphology and taxonomic affinities in cycadales. Am. J. Bot. 1988, 75, 1501–1516.
- Bolinder, K.; Niklas, K.J.; Rydin, C. Aerodynamics and pollen ultrastructure in Ephedra. Am. J. Bot. 2015, 102, 457–470.
- Rydin, C.; Hoorn, C. The Gnetales: Past and present. Grana 2016, 55, 1–4.
- Gelbart, G.; von Aderkas, P. Ovular secretions as part of pollination mechanisms in conifers. Ann. For. Sci. 2002, 59, 345–357.
- Coulter, A.; Poulis, B.A.D.; von Aderkas, P. Pollination drops as dynamic apoplastic secretions. Flora Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 482–490.
- Leslie, A.B. Flotation preferentially selects saccate pollen during conifer pollination. New Phytol. 2010, 188, 273–279.
- Lu, Y.; Jin, B.; Wang, L.; Wang, Y.; Wang, D.; Jiang, X.X.; Chen, P. Adaptation of male reproductive structures to wind pollination in gymnosperms: Cones and pollen grains. Can. J. Plant Sci. 2011, 91, 897–906.
- Franchi, G.G.; Piotto, B.; Nepi, M.; Baskin, C.C.; Baskin, J.M.; Pacini, E. Pollen and seed desiccation tolerance in relation to degree of developmental arrest, dispersal, and survival. J. Exp. Bot. 2011, 62, 5267–5281.
- Labandeira, C.C.; Kvaček, J.; Mostovski, M.B. Pollination drops, pollen, and insect pollination of Mesozoic gymnosperms. Taxon 2007, 56, 663–695.
- Klavins, S.D.; Kellogg, D.W.; Krings, M.; Taylor, E.L.; Taylor, T.N. Coprolites in a Middle Triassic cycad pollen cone: Evidence for insect pollination in early cycads? Evol. Ecol. Res. 2005, 7, 479–488.
- Nygaard, P. Studies on the Germination of Pine Pollen (Pinus mugo) in vitro. I. Growth Conditions and Effects of pH and Temperature on Germination, Tube Growth and Respiration. Physiol. Plant. 1969, 22, 338–346.
- Dawkins, M.D.; Owens, J.N. In vitro and in vivo pollen hydration, germination, and pollen-tube growth in white spruce, Picea glauca (Moench) Voss. Int. J. Plant Sci. 1993, 154, 506–521.
- Muren, R.C.; Ching, T.M.; Ching, K.I.M.K. Metabolic Study of Douglas-fir Pollen Germination in vitro. Physiol. Plant. 1979, 46, 287–292.
- Fernando, D.D.; Lazzaro, M.D.; Owens, J.N. Growth and development of conifer pollen tubes. Sex. Plant Reprod. 2005, 18, 149–162.
- Furness, C.A.; Rudall, P.J. Pollen aperture evolution—A crucial factor for eudicot success? Trends Plant Sci. 2004, 9, 154–158.
- Albert, B.; Ressayre, A.; Dillmann, C.; Carlson, A.L.; Swanson, R.J.; Gouyon, P.-H.; Dobritsa, A.A. Effect of aperture number on pollen germination, survival and reproductive success in Arabidopsis thaliana. Ann. Bot. 2018, 121, 733–740.
- Breygina, M.; Maksimov, N.; Polevova, S.; Evmenyeva, A. Bipolar pollen germination in blue spruce (Picea pungens). Protoplasma 2019, 256, 941–949.
- El-Ghazaly, G.; Rowley, J.; Hesse, M. Polarity, aperture condition and germination in pollen grains of Ephedra (Gnetales). Plant Syst. Evol. 1998, 213, 217–231.
- Surso, M. Pollination and pollen germination in common juniper (Juniperus communis: Cupressaceae). Arct. Environ. Res. 2018, 18, 162–174.
- Duhoux, E. Mechanism of exine rupture in hydrated ta×oid type of pollen. Grana 1982, 21, 1–7.
- Rydin, C.; Friis, E.M. Pollen germination in Welwitschia mirabilis Hook. f.: Differences between the polyplicate pollen producing genera of the Gnetales. Grana 2005, 44, 137–141.
- Prior, N.; Little, S.A.; Boyes, I.; Griffith, P.; Husby, C.; Pirone-Davies, C.; Stevenson, D.W.; Tomlinson, P.B.; von Aderkas, P. Complex reproductive secretions occur in all extant gymnosperm lineages: A proteomic survey of gymnosperm pollination drops. Plant Reprod. 2019, 32, 153–166.
- Little, S.; Prior, N.; Pirone, C.; von Aderkas, P. Pollen-ovule Interactions in Gymnosperms. In Reproductive Biology of Plants; CRC Press: Boca Raton, FL, USA, 2014; pp. 97–117.
- Prior, N.; Little, S.A.; Pirone, C.; Gill, J.E.; Smith, D.; Han, J.; Hardie, D.; O’Leary, S.J.B.; Wagner, R.E.; Cross, T. Application of proteomics to the study of pollination drops. Appl. Plant Sci. 2013, 1, 1300008.
- Losada, J.M.; Leslie, A.B. Why are the seed cones of conifers so diverse at pollination? Ann. Bot. 2018, 121, 1319–1331.
- Möller, M.; Mill, R.R.; Glidewell, S.M.; Masson, D.; Williamson, B.; Bateman, R.M. Comparative biology of the pollination mechanisms in Acmopyle pancheri and Phyllocladus hypophyllus (Podocarpaceae s. l.). Ann. Bot. 2000, 86, 149–158.
- Williams, J.H. Pollen Tube Growth Rates and the Diversification of Flowering Plant Reproductive Cycles. Int. J. Plant Sci. 2012, 173, 649–661.
