
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mária Bagyánszki | + 2144 word(s) | 2144 | 2021-06-01 08:57:47 |
Video Upload Options
Nitrergic enteric neurons are key players of the descending inhibitory reflex of intestinal peristalsis, therefore loss or damage of these neurons can contribute to developing gastrointestinal motility disturbances suffered by patients worldwide. There is accumulating evidence that the vulnerability of nitrergic enteric neurons to neuropathy is strictly region-specific and that the two main enteric plexuses display different nitrergic neuronal damage. Alterations both in the proportion of the nitrergic subpopulation and in the total number of enteric neurons suggest that modification of the neurochemical character or neuronal death occurs in the investigated gut segments.
1. Introduction
2. Nitrergic Enteric Neurons
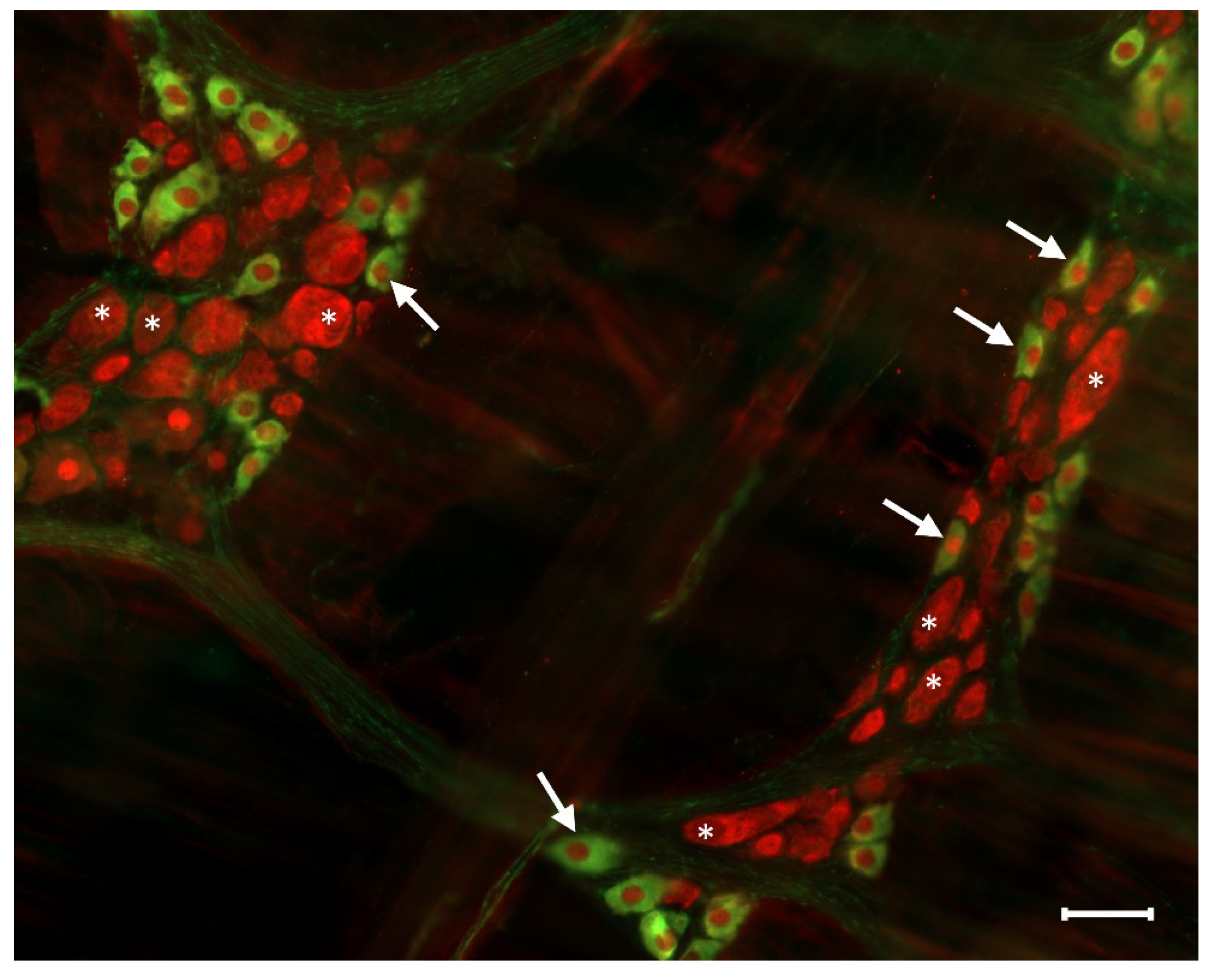
3. Pathophysiology of Nitrergic Enteric Neurons
3.1. Type 1 Diabetes
3.2. Chronic Alcohol Consumption
3.3. Intestinal Inflammation
3.4. Ischaemic Injuries
4. Conclusions
5. Abbreviations
|
AGE |
advanced glycation end product |
|
eNOS |
endothelial nitric oxide synthase |
|
ENS |
enteric nervous system |
|
GI |
gastrointestinal |
|
IBS |
irritable bowel syndrome |
|
iNOS |
inducible nitric oxide synthase |
|
I/R |
ischemia/reperfusion |
|
NANC |
non-adrenergic, non-cholinergic |
|
NO |
nitric oxide |
|
NOS |
nitric oxide synthase |
|
nNOS |
neuronal nitric oxide synthase |
|
VIP |
vasoactive intestinal polypeptide |
References
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837.
- Wallace, J.L. Nitric oxide in the gastrointestinal tract: Opportunities for drug development. Br. J. Pharmacol. 2019, 176, 147–154.
- Bodi, N.; Battonyai, I.; Talapka, P.; Fekete, E.; Bagyanszki, M. Spatial pattern analysis of nitrergic neurons in the myenteric plexus of the duodenum of different mammalian species. Acta Biol. Hung. 2009, 60, 347–358.
- Sanders, K.M.; Ward, S.M. Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract. Br. J. Pharmacol. 2019, 176, 212–227.
- Rivera, L.R.; Poole, D.P.; Thacker, M.; Furness, J.B. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol. Motil. 2011, 23, 980–988.
- Furness, J.B. The organisation of the autonomic nervous system: Peripheral connections. Auton Neurosci. 2006, 130, 1–5.
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294.
- Lomax, A.E.; Linden, D.R.; Mawe, G.M.; Sharkey, K.A. Effects of gastrointestinal inflammation on enteroendocrine cells and enteric neural reflex circuits. Auton Neurosci. 2006, 126–127, 250–257.
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71.
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflamm. 2019, 16, 53.
- Wirth, R.; Bodi, N.; Maroti, G.; Bagyanszki, M.; Talapka, P.; Fekete, E.; Bagi, Z.; Kovacs, K.L. Regionally distinct alterations in the composition of the gut microbiota in rats with streptozotocin-induced diabetes. PLoS ONE 2014, 9, e110440.
- De Palma, G.; Collins, S.M.; Bercik, P.; Verdu, E.F. The microbiota-gut-brain axis in gastrointestinal disorders: Stressed bugs, stressed brain or both? J. Physiol. 2014, 592, 2989–2997.
- Furness, J.B. Types of neurons in the enteric nervous system. J. Auton Nerv. Syst. 2000, 81, 87–96.
- Qu, Z.D.; Thacker, M.; Castelucci, P.; Bagyanszki, M.; Epstein, M.L.; Furness, J.B. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008, 334, 147–161.
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell Physiol. 2017, 232, 2359–2372.
- Lefebvre, R.A. Nitric oxide in the peripheral nervous system. Ann. Med. 1995, 27, 379–388.
- Mizuta, Y.; Takahashi, T.; Owyang, C. Nitrergic regulation of colonic transit in rats. Am. J. Physiol. 1999, 277, G275–G279.
- Bodi, N.; Jancso, Z.; Talapka, P.; Pal, A.; Poles, M.Z.; Bagyanszki, M.; Hermesz, E.; Fekete, E. Gut region-specific rearrangement of the cellular and subcellular compartments of nitric oxide synthase isoforms after chronic ethanol consumption in rats. Histol. Histopathol. 2014, 29, 1547–1555.
- Bredt, D.S.; Glatt, C.E.; Hwang, P.M.; Fotuhi, M.; Dawson, T.M.; Snyder, S.H. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron 1991, 7, 615–624.
- Valentine, J.F.; Tannahill, C.L.; Stevenot, S.A.; Sallustio, J.E.; Nick, H.S.; Eaker, E.Y. Colitis and interleukin 1beta up-regulate inducible nitric oxide synthase and superoxide dismutase in rat myenteric neurons. Gastroenterology 1996, 111, 56–64.
- Vannucchi, M.G.; Corsani, L.; Bani, D.; Faussone-Pellegrini, M.S. Myenteric neurons and interstitial cells of Cajal of mouse colon express several nitric oxide synthase isoforms. Neurosci. Lett. 2002, 326, 191–195.
- Talapka, P.; Bodi, N.; Battonyai, I.; Fekete, E.; Bagyanszki, M. Subcellular distribution of nitric oxide synthase isoforms in the rat duodenum. World J. Gastroenterol. 2011, 17, 1026–1029.
- Bagyanszki, M.; Torfs, P.; Krecsmarik, M.; Fekete, E.; Adriaensen, D.; Van Nassauw, L.; Timmermans, J.P.; Kroese, A.B. Chronic alcohol consumption induces an overproduction of NO by nNOS- and iNOS-expressing myenteric neurons in the murine small intestine. Neurogastroenterol. Motil. 2011, 23, e237–e248.
- Mongardi Fantaguzzi, C.; Thacker, M.; Chiocchetti, R.; Furness, J.B. Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res. 2009, 336, 179–189.
- Noorian, A.R.; Taylor, G.M.; Annerino, D.M.; Greene, J.G. Neurochemical phenotypes of myenteric neurons in the rhesus monkey. J. Comp. Neurol. 2011, 519, 3387–3401.
- Cellek, S. Point of NO return for nitrergic nerves in diabetes: A new insight into diabetic complications. Curr. Pharm. Des. 2004, 10, 3683–3695.
- Izbeki, F.; Wittman, T.; Rosztoczy, A.; Linke, N.; Bodi, N.; Fekete, E.; Bagyanszki, M. Immediate insulin treatment prevents gut motility alterations and loss of nitrergic neurons in the ileum and colon of rats with streptozotocin-induced diabetes. Diabetes Res. Clin. Pract. 2008, 80, 192–198.
- Demedts, I.; Masaoka, T.; Kindt, S.; De Hertogh, G.; Geboes, K.; Farre, R.; Vanden Berghe, P.; Tack, J. Gastrointestinal motility changes and myenteric plexus alterations in spontaneously diabetic biobreeding rats. J. Neurogastroenterol. Motil. 2013, 19, 161–170.
- Watkins, C.C.; Sawa, A.; Jaffrey, S.; Blackshaw, S.; Barrow, R.K.; Snyder, S.H.; Ferris, C.D. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J. Clin. Investig. 2000, 106, 803.
- Giancola, F.; Fracassi, F.; Gallucci, A.; Sadeghinezhad, J.; Polidoro, G.; Zini, E.; Asti, M.; Chiocchetti, R. Quantification of nitrergic neurons in the myenteric plexus of gastric antrum and ileum of healthy and diabetic dogs. Auton Neurosci. 2016, 197, 25–33.
- Miller, S.M.; Narasimhan, R.A.; Schmalz, P.F.; Soffer, E.E.; Walsh, R.M.; Krishnamurthi, V.; Pasricha, P.J.; Szurszewski, J.H.; Farrugia, G. Distribution of interstitial cells of Cajal and nitrergic neurons in normal and diabetic human appendix. Neurogastroenterol. Motil. 2008, 20, 349–357.
- Zhang, C.M.; Huang, X.; Lu, H.L.; Meng, X.M.; Song, N.N.; Chen, L.; Kim, Y.C.; Chen, J.; Xu, W.X. Diabetes-induced damage of gastric nitric oxide neurons mediated by P2×7R in diabetic mice. Eur. J. Pharmacol. 2019, 851, 151–160.
- Yoneda, S.; Kadowaki, M.; Kuramoto, H.; Fukui, H.; Takaki, M. Enhanced colonic peristalsis by impairment of nitrergic enteric neurons in spontaneously diabetic rats. Auton Neurosci. 2001, 92, 65–71.
- Bodi, N.; Szalai, Z.; Chandrakumar, L.; Bagyanszki, M. Region-dependent effects of diabetes and insulin-replacement on neuronal nitric oxide synthase- and heme oxygenase-immunoreactive submucous neurons. World J. Gastroenterol. 2017, 23, 7359–7368.
- Bodi, N.; Talapka, P.; Poles, M.Z.; Hermesz, E.; Jancso, Z.; Katarova, Z.; Izbeki, F.; Wittmann, T.; Fekete, E.; Bagyanszki, M. Gut region-specific diabetic damage to the capillary endothelium adjacent to the myenteric plexus. Microcirculation 2012, 19, 316–326.
- Bagyanszki, M.; Bodi, N. Diabetes-related alterations in the enteric nervous system and its microenvironment. World J. Diabetes 2012, 3, 80–93.
- Gangula, P.R.; Maner, W.L.; Micci, M.A.; Garfield, R.E.; Pasricha, P.J. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G725–G733.
- Gangula, P.R.; Mukhopadhyay, S.; Ravella, K.; Cai, S.; Channon, K.M.; Garfield, R.E.; Pasricha, P.J. Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G692–G699.
- Cellek, S.; Foxwell, N.A.; Moncada, S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes 2003, 52, 2353–2362.
- Cellek, S.; Qu, W.; Schmidt, A.M.; Moncada, S. Synergistic action of advanced glycation end products and endogenous nitric oxide leads to neuronal apoptosis in vitro: A new insight into selective nitrergic neuropathy in diabetes. Diabetologia 2004, 47, 331–339.
- Korenaga, K.; Micci, M.A.; Taglialatela, G.; Pasricha, P.J. Suppression of nNOS expression in rat enteric neurones by the receptor for advanced glycation end-products. Neurogastroenterol. Motil. 2006, 18, 392–400.
- Jeyabal, P.V.; Kumar, R.; Gangula, P.R.; Micci, M.A.; Pasricha, P.J. Inhibitors of advanced glycation end-products prevent loss of enteric neuronal nitric oxide synthase in diabetic rats. Neurogastroenterol. Motil. 2008, 20, 253–261.
- Chandrakumar, L.; Bagyanszki, M.; Szalai, Z.; Mezei, D.; Bodi, N. Diabetes-Related Induction of the Heme Oxygenase System and Enhanced Colocalization of Heme Oxygenase 1 and 2 with Neuronal Nitric Oxide Synthase in Myenteric Neurons of Different Intestinal Segments. Oxid. Med. Cell Longev. 2017, 2017, 1890512.
- Shotton, H.R.; Lincoln, J. Diabetes only affects nitric oxide synthase-containing myenteric neurons that do not contain heme oxygenase 2. Brain Res. 2006, 1068, 248–256.
- Krecsmarik, M.; Izbeki, F.; Bagyanszki, M.; Linke, N.; Bodi, N.; Kaszaki, J.; Katarova, Z.; Szabo, A.; Fekete, E.; Wittmann, T. Chronic ethanol exposure impairs neuronal nitric oxide synthase in the rat intestine. Alcohol Clin. Exp. Res. 2006, 30, 967–973.
- Bagyanszki, M.; Bodi, N. Gut region-dependent alterations of nitrergic myenteric neurons after chronic alcohol consumption. World J. Gastrointest. Pathophysiol. 2015, 6, 51–57.
- Bagyanszki, M.; Krecsmarik, M.; De Winter, B.Y.; De Man, J.G.; Fekete, E.; Pelckmans, P.A.; Adriaensen, D.; Kroese, A.B.; Van Nassauw, L.; Timmermans, J.P. Chronic alcohol consumption affects gastrointestinal motility and reduces the proportion of neuronal NOS-immunoreactive myenteric neurons in the murine jejunum. Anat. Rec. 2010, 293, 1536–1542.
- Bonthius, D.J.; Bonthius, N.E.; Li, S.; Karacay, B. The protective effect of neuronal nitric oxide synthase (nNOS) against alcohol toxicity depends upon the NO-cGMP-PKG pathway and NF-kappaB. Neurotoxicology 2008, 29, 1080–1091.
- Karacay, B.; Bonthius, D.J. The neuronal nitric oxide synthase (nNOS) gene and neuroprotection against alcohol toxicity. Cell Mol. Neurobiol 2015, 35, 449–461.
- Talapka, P.; Nagy, L.I.; Pal, A.; Poles, M.Z.; Berko, A.; Bagyanszki, M.; Puskas, L.G.; Fekete, E.; Bodi, N. Alleviated mucosal and neuronal damage in a rat model of Crohn’s disease. World J. Gastroenterol. 2014, 20, 16690–16697.
- Marlow, S.L.; Blennerhassett, M.G. Deficient innervation characterizes intestinal strictures in a rat model of colitis. Exp. Mol. Pathol. 2006, 80, 54–66.
- Boyer, L.; Sidpra, D.; Jevon, G.; Buchan, A.M.; Jacobson, K. Differential responses of VIPergic and nitrergic neurons in paediatric patients with Crohn’s disease. Auton Neurosci. 2007, 134, 106–114.
- Winston, J.H.; Li, Q.; Sarna, S.K. Paradoxical regulation of ChAT and nNOS expression in animal models of Crohn’s colitis and ulcerative colitis. Am. J. Physiol. Gastrointest. Liver Physiol 2013, 305, G295–G302.
- Li, S.; Fei, G.; Fang, X.; Yang, X.; Sun, X.; Qian, J.; Wood, J.D.; Ke, M. Changes in Enteric Neurons of Small Intestine in a Rat Model of Irritable Bowel Syndrome with Diarrhea. J. Neurogastroenterol. Motil. 2016, 22, 310–320.
- Rahman, A.A.; Robinson, A.M.; Jovanovska, V.; Eri, R.; Nurgali, K. Alterations in the distal colon innervation in Winnie mouse model of spontaneous chronic colitis. Cell Tissue Res. 2015, 362, 497–512.
- Schneider, J.; Jehle, E.C.; Starlinger, M.J.; Neunlist, M.; Michel, K.; Hoppe, S.; Schemann, M. Neurotransmitter coding of enteric neurones in the submucous plexus is changed in non-inflamed rectum of patients with Crohn’s disease. Neurogastroenterol. Motil. 2001, 13, 255–264.
- de Fontgalland, D.; Brookes, S.J.; Gibbins, I.; Sia, T.C.; Wattchow, D.A. The neurochemical changes in the innervation of human colonic mesenteric and submucosal blood vessels in ulcerative colitis and Crohn’s disease. Neurogastroenterol. Motil. 2014, 26, 731–744.
- Sanovic, S.; Lamb, D.P.; Blennerhassett, M.G. Damage to the enteric nervous system in experimental colitis. Am. J. Pathol. 1999, 155, 1051–1057.
- Venkataramana, S.; Lourenssen, S.; Miller, K.G.; Blennerhassett, M.G. Early inflammatory damage to intestinal neurons occurs via inducible nitric oxide synthase. Neurobiol. Dis. 2015, 75, 40–52.
- Turco, F.; Sarnelli, G.; Cirillo, C.; Palumbo, I.; De Giorgi, F.; D’Alessandro, A.; Cammarota, M.; Giuliano, M.; Cuomo, R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut 2014, 63, 105–115.
- Brown, I.A.; McClain, J.L.; Watson, R.E.; Patel, B.A.; Gulbransen, B.D. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol. Gastroenterol. Hepatol. 2016, 2, 77–91.
- Rachmilewitz, D.; Stamler, J.S.; Bachwich, D.; Karmeli, F.; Ackerman, Z.; Podolsky, D.K. Enhanced colonic nitric oxide generation and nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Gut 1995, 36, 718–723.
- Ljung, T.; Lundberg, S.; Varsanyi, M.; Johansson, C.; Schmidt, P.T.; Herulf, M.; Lundberg, J.O.; Hellstrom, P.M. Rectal nitric oxide as biomarker in the treatment of inflammatory bowel disease: Responders versus nonresponders. World J. Gastroenterol. 2006, 12, 3386–3392.
- Rychlik, A.; Gonkowski, S.; Nowicki, M.; Calka, J. Inflammatory bowel disease affects density of nitrergic nerve fibers in the mucosal layer of the canine gastrointestinal tract. Can. J. Vet. Res. 2017, 81, 129–136.
- Wedel, T.; Krammer, H.J.; Kuhnel, W.; Sigge, W. Alterations of the enteric nervous system in neonatal necrotizing enterocolitis revealed by whole-mount immunohistochemistry. Pediatr. Pathol. Lab. Med. 1998, 18, 57–70.
- Lindestrom, L.M.; Ekblad, E. Structural and neuronal changes in rat ileum after ischemia with reperfusion. Dig. Dis. Sci. 2004, 49, 1212–1222.
- Mei, F.; Guo, S.; He, Y.T.; Zhu, J.; Zhou, D.S.; Niu, J.Q.; Wang, H.Z.; Tian, Y.P. Apoptosis of interstitial cells of Cajal, smooth muscle cells, and enteric neurons induced by intestinal ischemia and reperfusion injury in adult guinea pigs. Virchows Arch. 2009, 454, 401–409.
- Rivera, L.R.; Pontell, L.; Cho, H.J.; Castelucci, P.; Thacker, M.; Poole, D.P.; Frugier, T.; Furness, J.B. Knock out of neuronal nitric oxide synthase exacerbates intestinal ischemia/reperfusion injury in mice. Cell Tissue Res. 2012, 349, 565–576.
- Rivera, L.R.; Thacker, M.; Castelucci, P.; Bron, R.; Furness, J.B. The reactions of specific neuron types to intestinal ischemia in the guinea pig enteric nervous system. Acta Neuropathol. 2009, 118, 261–270.
- da Silva de Souza, A.C.; Borges, S.C.; Beraldi, E.J.; de Sa-Nakanishi, A.B.; Comar, J.F.; Bracht, A.; Natali, M.R.; Buttow, N.C. Resveratrol Reduces Morphologic Changes in the Myenteric Plexus and Oxidative Stress in the Ileum in Rats with Ischemia/Reperfusion Injury. Dig. Dis. Sci. 2015, 60, 3252–3263.
- Marosti, A.R.; da Silva, M.V.; Palombit, K.; Mendes, C.E.; Tavares-de-Lima, W.; Castelucci, P. Differential effects of intestinal ischemia and reperfusion in rat enteric neurons and glial cells expressing P2 × 2 receptors. Histol. Histopathol. 2015, 30, 489–501.
- Rivera, L.R.; Thacker, M.; Pontell, L.; Cho, H.J.; Furness, J.B. Deleterious effects of intestinal ischemia/reperfusion injury in the mouse enteric nervous system are associated with protein nitrosylation. Cell Tissue Res. 2011, 344, 111–123.
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269.
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526.
- Joca, S.R.L.; Sartim, A.G.; Roncalho, A.L.; Diniz, C.F.A.; Wegener, G. Nitric oxide signalling and antidepressant action revisited. Cell Tissue Res. 2019.





