1. Introduction
The environment is a very dynamic system which is shaped by various factors, including temperature, pressure, water or wind erosion processes, pH, redox potential, and human activity. Various substances introduced by humans to the environment may determine changes taking place in nature, affecting organisms, disrupting their natural functions. Contamination occurs when a dangerous substance is introduced into the environment or present in a given element of the environment in concentration, form, or nature, that violates the natural system of the environment, exceeds the applicable regulations, and poses a threat to human health. It should be added that contamination of the environment may be caused by natural processes taking place in the environment, such as volcanic eruptions, but most often it is caused by human activity, such as technological line failure, sewage discharge, leachate from landfills, and incineration in uncontrolled conditions.
Depending on the source of emission and the type of contamination, the migration routes in the environment and the impacts on individual elements may be different. The variety and number of sources of pollutant emissions to the environment and the type of the introduced substance can often cause irreversible or almost irreversible changes in nature. Therefore, chemical, biological, radiological, or nuclear (CBRN) events require appropriate measures to minimize their negative impact.
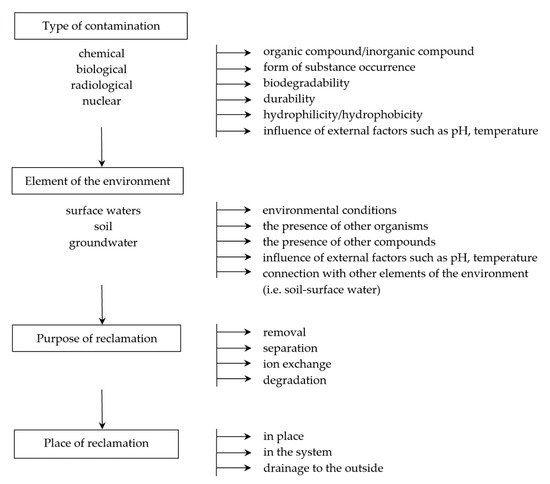
In the case of contamination, it is very important to select the appropriate techniques and tools that minimize the negative impact and make it possible to remove the substance or organism (in the case of biological contamination) from the system. Products that may be generated during environmental remediation should also be considered so that they do not present a greater risk than the substance to be neutralized. Tools and techniques must therefore be adapted to the type of environment, taking into account physicochemical and biological conditions, including diffusion process, temperature and pressure conditions, as well as the natural presence of organisms ().
Figure 1. An example of a decision-making path necessary for the correct selection of the technique and type of membrane.
2. Application of Membranes in Contamination Situations
Potential emerging pollutants, such as hazardous chemicals, toxic metals, bio-waste, etc., pose a serious threat to health, hygiene, and ecology by polluting the environment. These pollutants from various sources, including industrial wastewater, mainly from the pharmaceutical, food, and metal processing industries, can contaminate water and disrupt aquatic ecosystems. The discharged wastewater at the source requires clear identification, separation and disposal, otherwise, it can pose serious problems for water quality and ecology in general. Conventional water treatment methods, such as adsorption, bio-oxidation, coagulation, sedimentation, and filtration as well as hybrid methods, such as chlorination and UV irradiation, have been widely described in the literature, although most of these approaches are insufficient for effective wastewater treatment
[1].
On the other hand, water treatment by membrane-based separation processes is quite expensive and energy-intensive compared to other conventional treatment technologies. However, membrane treatment processes have several clear advantages, such as the production of high-quality water with a high recovery rate of valuable chemicals/metals and low maintenance costs
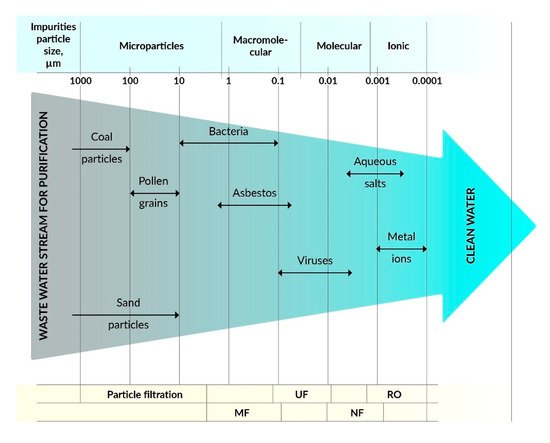
[2][3]. The most commonly used methods are microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), reverse osmosis (RO), and forward osmosis (FO), and some hybrid technologies such as membrane bioreactors (MBR) and photocatalytic membrane reactors (PMR). Membrane processes depend on the type of membranes that are made of various materials, including polymers, ceramics, zeolites, etc., with specific filtration properties. The effectiveness of membranes depends on the surface charge, pore size, membrane morphology, and hydrophobic/hydrophilic properties. Polymer membranes can be used in various filtration methods, such as MF, UF, NF, RO, and FO depending on pore size and morphology as well as specific separation needs (see ).
Figure 2. Dependence of filtration methods on the type of pollutants.
The above filtration methods use various separation techniques, e.g., solution diffusion or molecular diffusion or the size exclusive principle
[4].
The membranes used for MF have larger pore sizes (0.1–5 µm) than the membranes used for UF, which are typically used to separate contamination with a particle size in the range of 0.1–10 µm. On the other hand, UF membranes with pore sizes from 0.01 to 0.1 μm can be used to separate colloidal particles, macromolecules, biopolymers, and viruses, whose sizes are usually in the range from 0.01 to 0.2 μm, and the process uses the principle of size exclusion. Commercially, UF is widely used for wastewater treatment, recovery of surfactants from industrial line washing, and in food processing and protein separation, etc. UF membranes are made of cellulose derivatives, inorganic materials, such as TiO
2, Al
2O
3, ZrO, as well as from common polymers, such as poly(acrylonitrile) (PAN), poly(sulfonamide) (PSA), poly(ether sulfone) (PES), poly(vinylidene fluoride) (PVDF), etc.
[5][6].
NF membranes enable the separation of particles in the size range of 0.001–0.01 µm, which include most organic compounds, biomacromolecules, and various metal salts (except divalent salts). The capacity of NF is between RO and UF
[7]. RO membranes are non-porous, made of solid polymers with voids, free-volume channels, or pore sizes ranging from ∼0.0001 to 0.001 μm
[8]. RO membranes separate low molecular weight inorganic components, including metal ions. The most common applications of RO are the treatment of wastewater from pulp and paper mills to produce drinking water
[9].
In recent years, ceramic or zeolite composite membranes have emerged as high-performance RO and NF membranes that have been successfully commercialized for the separation of pollutants
[10]. These membranes are generally made of composite materials and contain various fibers such as hollow or structural fibers, or sheet nanostructures such as graphene and layered silicates in the polymer matrix
[11]. Several problems with the use of RO membranes, such as their high energy requirements and fouling of membrane surfaces, have resulted in the development of techniques
[12] and FO membranes in which the osmotic gradient across the membrane plays an important role in mass transport and separation
[13]. FO is more suitable and energy efficient for the treatment of membrane fouling wastewater (e.g., landfill leachate) which may not be economical for RO. Initially, FO was regarded as an effective pretreatment step for subsequent processes in which purified water could be recovered from the dilute solution
[14]. However, FO as a single filter technique also finds some niche applications, such as diluting fertilizers and thickening fruit juices
[15].
3. Conclusions
Research on the use of polymer membranes to counteract the risk of environmental contamination is undergoing constant development due to the possibility of using this technology to purify a whole range of surface and ground waters as well as the soil environment, which is particularly important from the point of view of environmental protection.
In addition, the ability to recover valuable natural resources draws attention to important economic aspects. Increased interest in the use of this type of technology is also the result of the growing environmental awareness of societies. Polymer membrane processes do not require dosing of chemicals and do not transform pollutants, saving resources, energy, and human labor. In view of the above facts, there is a continuous and intensive development of research on obtaining more effective methods, allowing the modification of polymer membranes, and at the same time changing their physicochemical properties.
There are many advantages of using polymer membranes in environmental protection. First of all, compared to many other traditional techniques and processes, e.g., distillation, polymer membranes use less energy, as well as fewer raw materials and operating personnel. It should also be noted that the use of membrane techniques by industry may contribute to increasing resources (mainly water) and the reduction of the amount of solid and liquid waste generated in the production process, and thus allows for obtaining tangible economic benefits. Water purified by membrane processes can return to production and does not remain in the soil and groundwater, poisoning these resources.
In conclusion, the use of polymer membranes seems to be justified due to many factors, chiefly technical and economic (at least relatively low operating costs), as well as the properties of their easy adaptation in the environment. The introduction of membrane techniques into widespread use is considered to be the right step in the field of environmental protection. It is also assessed that these technologies in water and soil treatment applications are currently among the best available technologies and that they make a beneficial contribution to environmental sustainability. Some of them require relatively high investment outlays. The applicable legal regulations and economic instruments of environmental policy should support the use of these techniques in industry and the economy. For example, the penalties related to the direct introduction of pollutants into the environment are high and it remains necessary to invest in environmental protection, including investments in polymer membrane techniques.