Depending on the type of contamination, various methods are used, including sorption, biodegradation, separation, or ion exchange processes in which membranes play an important role. The type of membrane is selected in respect of both the environment and the type of neutralized pollutants.
- polymer membranes
- application
- CBRN contamination
- remediation
1. Introduction
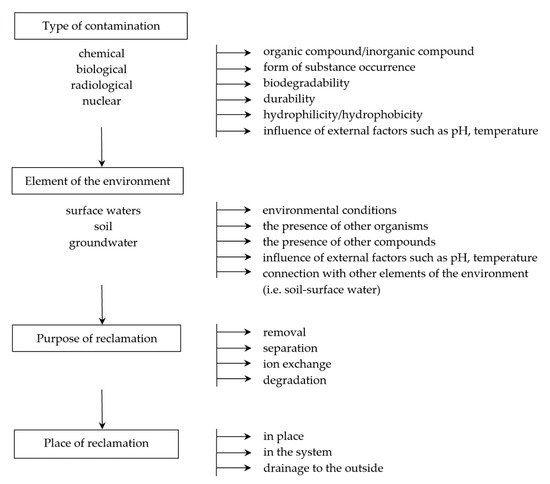
2. Application of Membranes in Contamination Situations
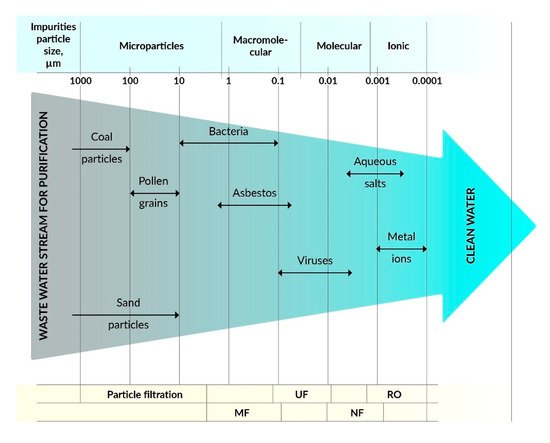
Research on the use of polymer membranes to counteract the risk of environmental contamination is undergoing constant development due to the possibility of using this technology to purify a whole range of surface and ground waters as well as the soil environment, which is particularly important from the point of view of environmental protection.
In addition, the ability to recover valuable natural resources draws attention to important economic aspects. Increased interest in the use of this type of technology is also the result of the growing environmental awareness of societies. Polymer membrane processes do not require dosing of chemicals and do not transform pollutants, saving resources, energy, and human labor. In view of the above facts, there is a continuous and intensive development of research on obtaining more effective methods, allowing the modification of polymer membranes, and at the same time changing their physicochemical properties.
There are many advantages of using polymer membranes in environmental protection. First of all, compared to many other traditional techniques and processes, e.g., distillation, polymer membranes use less energy, as well as fewer raw materials and operating personnel. It should also be noted that the use of membrane techniques by industry may contribute to increasing resources (mainly water) and the reduction of the amount of solid and liquid waste generated in the production process, and thus allows for obtaining tangible economic benefits. Water purified by membrane processes can return to production and does not remain in the soil and groundwater, poisoning these resources.
Research on the use of polymer membranes to counteract the risk of environmental contamination is undergoing constant development due to the possibility of using this technology to purify a whole range of surface and ground waters as well as the soil environment, which is particularly important from the point of view of environmental protection.
In addition, the ability to recover valuable natural resources draws attention to important economic aspects. Increased interest in the use of this type of technology is also the result of the growing environmental awareness of societies. Polymer membrane processes do not require dosing of chemicals and do not transform pollutants, saving resources, energy, and human labor. In view of the above facts, there is a continuous and intensive development of research on obtaining more effective methods, allowing the modification of polymer membranes, and at the same time changing their physicochemical properties.
There are many advantages of using polymer membranes in environmental protection. First of all, compared to many other traditional techniques and processes, e.g., distillation, polymer membranes use less energy, as well as fewer raw materials and operating personnel. It should also be noted that the use of membrane techniques by industry may contribute to increasing resources (mainly water) and the reduction of the amount of solid and liquid waste generated in the production process, and thus allows for obtaining tangible economic benefits. Water purified by membrane processes can return to production and does not remain in the soil and groundwater, poisoning these resources.
References
- Jones, O.A.H.; Voulvoulis, N.; Lester, J.N. Human Pharmaceuticals in Wastewater Treatment Processes. Crit. Rev. Environ. Sci. Technol. 2005, 35, 401–427.
- Mohammad, A.; Teow, Y.; Ang, W.; Chung, Y.; Oatley-Radcliffe, D.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254.
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane bioreactors—A review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Membr. Sci. 2017, 527, 207–227.
- Alzahrani, S.; Mohammad, A.W. Challenges and trends in membrane technology implementation for produced water treatment: A review. J. Water Process. Eng. 2014, 4, 107–133.
- Wang, J.; Myers, E. Tidal Datum Changes Induced by Morphological Changes of North Carolina Coastal Inlets. J. Mar. Sci. Eng. 2016, 4, 79.
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42.
- Nataraj, S.; Hosamani, K.; Aminabhavi, T. Nanofiltration and reverse osmosis thin film composite membrane module for the removal of dye and salts from the simulated mixtures. Desalination 2009, 249, 12–17.
- Nataraj, S.K.; Hosamani, K.M.; Aminabhavi, T.M. Distillery wastewater treatment by the membrane-based nanofiltration and reverse osmosis processes. Water Res. 2006, 40, 2349–2356.
- Fane, A.; Tang, C.; Wang, R. Membrane Technology for Water: Microfiltration, Ultrafiltration, Nanofiltration, and Reverse Osmosis. In Treatise on Water Science; Elsevier BV: Amsterdam, The Netherlands, 2011; pp. 301–335.
- Mandegari, M.; Fashandi, H. Untapped potentials of acrylonitrile-butadiene-styrene/polyurethane (ABS/PU) blend membrane to purify dye wastewater. J. Environ. Manag. 2017, 197, 464–475.
- Aminabhavi, T.; Mallikarjuna, N. Column: Polymeric Membranes. Polym. News 2004, 29, 193–195.
- Hoek, E.M.; Ghosh, A.K.; Huang, X.; Liong, M.; Zink, J.I. Physical–chemical properties, separation performance, and fouling resistance of mixed-matrix ultrafiltration membranes. Desalination 2011, 283, 89–99.
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21.
- Achilli, A.; Cath, T.; Marchand, E.A.; Childress, A.E. The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes. Desalination 2009, 239, 10–21.
- Chanukya, B.; Rastogi, N.K. Ultrasound assisted forward osmosis concentration of fruit juice and natural colorant. Ultrason. Sonochem. 2017, 34, 426–435.
