
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raghad AL-Ishaq | + 3982 word(s) | 3982 | 2021-06-03 10:19:16 | | | |
| 2 | Vivi Li | Meta information modification | 3982 | 2021-06-10 11:57:30 | | |
Video Upload Options
Gastrointestinal (GI) cancer is a prevailing global health disease with a high incidence rate which varies by region. It is a huge economic burden on health care providers. GI cancer affects different organs in the body such as the gastric organs, colon, esophagus, intestine, and pancreas. Phytochemicals are non-nutritive bioactive secondary compounds abundantly found in fruits, grains, and vegetables. Consumption of phytochemicals may protect against chronic diseases like cardiovascular disease, neurodegenerative disease, and cancer. Multiple studies have assessed the chemoprotective effect of selected phytochemicals in GI cancer, offering support to their potential towards reducing the pathogenesis of the disease.The aim of this review is to summarize the current knowledge addressing the anti-cancerous effects of selected dietary phytochemicals on GI cancer and their molecular activities on selected mechanisms, i.e., nuclear factor kappa-light-chain-enhancer of activated B cells (NF-B), detoxification enzymes, adenosine monophosphate activated protein kinase (AMPK), wingless-related integration site/-catenin (wingless-related integration site (Wnt)-catenin, cell apoptosis, phosphoinositide 3-kinases (PI3K)/ protein kinase B AKT/ mammalian target of rapamycin (mTOR), and mitogen-activated protein kinase (MAPK). Overall, phytochemicals improve cancer prognosis through the downregulation of -catenin phosphorylation, therefore enhancing apoptosis, and upregulation of the AMPK pathway, which supports cellular homeostasis. Nevertheless, more studies are needed to provide a better understanding of the mechanism of cancer treatment using phytochemicals and possible side effects associated with this approach.
1. Gastrointestinal Cancer and Phytochemicals
1.1. Gastrointestinal Cancer
1.2. Colorectal Cancer
1.3. Esophageal Cancer
1.4. Diet and Microbial Metabolites
2. Anti-Cancerous Effects of Selected Phytochemicals
2.1. Carotenoids
2.1.1. Lutein
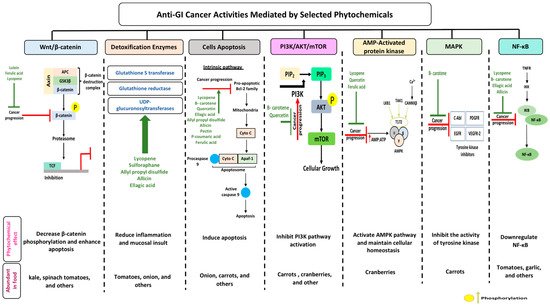
2.1.2. Lycopene
2.2. Proanthocyanidins
2.2.1. Quercetin
2.2.2. Ellagic Acid
2.3. Organosulfur Compounds
2.3.1. Allicin
2.3.2. Allyl Propyl Disulfide
2.3.3. Asparagusic Acid
2.3.4. Sulforaphane
| Phytochemical Subclass | Phytochemical and Structure | Dietary Source | Conversion Reaction | Metabolites Produced | Mechanism of Action | Model Used | References | |
|---|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | |||||||
| Carotenoids | Lutein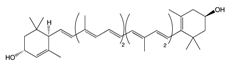 |
Egg yolk, kale, spinach, parsley, and peas | Oxidation | 3′-dehydro-lutein |
|
|
|
[42][46] |
Lycopene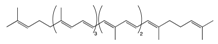 |
Tomato, guava, papaya, grapefruit, and watermelon | Auto-oxidation Radical-mediated oxidation | Apo-10′-lycopenoids |
|
|
|
[54][57] | |
β-Carotene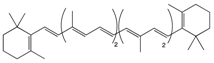 |
Carrot | Oxidation | Falcarindiol 6-methoxymellein |
|
|
|
[129][130][131] | |
| Pro-anthocy-anidins | Quercetin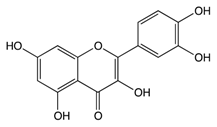 |
Cranberry | Sulfation Conjugation | 3-(4hydroxyphenyl) -propionic acid hippuric acid catechol-O-sulfate |
|
|
|
[68][69] |
Ellagic Acid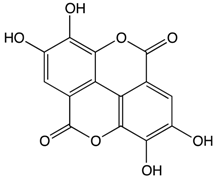 |
Bilberry | Glucuronidation O-methylation |
Peonidin-3-galactoside |
|
|
|
[80][81] | |
| Organosulfur Compounds | Allicin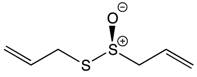 |
Garlic | Oxidation Hydrolysis | Allyl methyl sulfide (AMS) Allyl methyl sulfoxide (AMSO) Allyl methyl sulfone (AMSO2) |
|
|
|
[95][96] |
Allyl propyl disulfide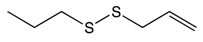 |
Onion | Reduction | Quercetin 3,4‘-diglucoside Quercetin 4‘-glucoside |
|
|
[106][107] | ||
Asparagusic acid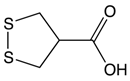 |
Asparagus | Sulfation | Asparagus polysaccharide dimethyl sulfide |
|
|
[112][113] | ||
Sulforaphane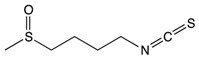 |
Broccoli, cabbage, Brussels sprout, and cauliflower | Hydrolysis | Thiocyanates Isothiocyanates Epithionitrile nitrile |
|
|
[124][125] | ||
| Other Phytochemicals | Pectin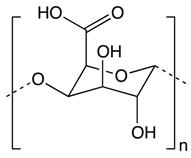 |
Apples, plums, oranges, and gooseberries | Colonic fermentation | Butyrate |
|
|
|
[132][133] |
Curcumin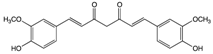 |
Ginger | Hydrolysis | Curcumin glucuronide Curcumin sulfate |
|
|
[134][135] | ||
p-Couramic acid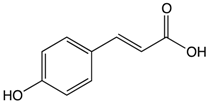 |
Navy beans | Hydrolysis | N-methylpipecolate 2-aminoadipate Piperidine Vanillate |
|
|
[136][137] | ||
Ferulic acid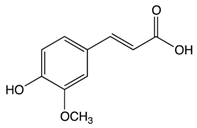 |
Rice bran | Colonic fermentation | Tryptophan α-ketoglutarate γ-tocopherol/β-tocopherol γ-tocotrienol |
|
|
|
[138][139] | |
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90.
- National Cancer Institute. Gastric cancer treatment PDQ. Available online: (accessed on 8 July 2010).
- Derakhshan, M.H.; Yazdanbod, A.; Sadjadi, A.R.; Shokoohi, B.; McColl, K.E.; Malekzadeh, R. High incidence of adenocarcinoma arising from the right side of the gastric cardia in NW Iran. Gut 2004, 53, 1262–1266.
- Zali, H.; Rezaei-Tavirani, M.; Azodi, M. Gastric cancer: Prevention, risk factors and treatment. Gastroenterol. Hepatol. Bed Bench. 2011, 4, 175–185.
- Sitarz, R.; Skierucha, M.; Mielko, J.; Offerhaus, G.J.A.; Maciejewski, R.; Polkowski, W.P. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018, 10, 239–248.
- Holian, O.; Wahid, S.; Atten, M.J.; Attar, B.M. Inhibition of gastric cancer cell proliferation by resveratrol: Role of nitric oxide. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G809–G816.
- Zhou, X.M.; Wong, B.C.; Fan, X.M.; Zhang, H.B.; Lin, M.C.; Kung, H.F.; Lam, S.K. Non-steroidal anti-inflammatory drugs induce apoptosis in gastric cancer cells through up-regulation of bax and bak. Carcinogenesis 2011, 22, 1393–1397.
- Hundahl, S.A.; Phillips, J.L.; Menck, H.R. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer 2000, 88, 921–932.
- Correa, P. Gastric cancer: Overview. Gastroenterol. Clin. North. Am. 2013, 211–217.
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386.
- Zali, H.; Rezaei-Tavirani, M.; Kariminia, A.; Yousefi, R.; Shokrgozar, M.A. Evaluation of growth inhibitory and apoptosis inducing activity of human calprotectin on the human gastric cell line (AGS). Iran. Biomed. J. 2008, 12, 7–14.
- Li, Y.H.; Niu, Y.B.; Sun, Y.; Zhang, F.; Liu, C.X.; Fan, L.; Mei, Q.B. Role of phytochemicals in colorectal cancer prevention. World J. Gastroenterol. 2015, 21, 9262–9272.
- Perdue, D.G.; Haverkamp, D.; Perkins, C.; Daley, C.M.; Provost, E. Geographic variation in colorectal cancer incidence and mortality, age of onset, and stage at diagnosis among American Indian and Alaska Native people, 1990–2009. Am. J. Public Health 2014, 104, S404–S414.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109.
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics. CA Cancer J. Clin. 2017, 67, 177–193.
- Tomasetti, C.; Marchionni, L.; Nowak, M.A.; Parmigiani, G.; Vogelstein, B. Only three driver gene mutations are required for the development of lung and colorectal cancers. Proc. Natl. Acad. Sci. USA 2015, 112, 118–123.
- Li, Y.; Zhang, T.; Chen, G.Y. Flavonoids and Colorectal Cancer Prevention. Antioxid 2018, 7, 187.
- Zhao, Y.; Hu, X.; Zuo, X.; Wang, M. Chemopreventive effects of some popular phytochemicals on human colon cancer: A review. Food Funct. 2018, 9, 4548–4568.
- Marmol, I.; Sanchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197.
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers. 2015, 1, 15065.
- Lambert, R.; Hainaut, P. The multidisciplinary management of gastrointestinal cancer. Epidemiology of oesophagogastric cancer. Best Pr. Res. Clin. Gastroenterol. 2007, 21, 921–945.
- Herszenyi, L.; Tulassay, Z. Epidemiology of gastrointestinal and liver tumors. Eur. Rev. Med. Pharm. Sci. 2010, 14, 249–258.
- Hongo, M.; Nagasaki, Y.; Shoji, T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J. Gastroenterol. Hepatol. 2009, 24, 729–735.
- Kubo, A.; Corley, D.A. Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am. J. Gastroenterol. 2004, 99, 582–588.
- Haidry, R.J.; Butt, M.A.; Dunn, J.M.; Gupta, A.; Lipman, G.; Smart, H.L.; Registry, U.R. Improvement over time in outcomes for patients undergoing endoscopic therapy for Barrett’s oesophagus-related neoplasia: 6-year experience from the first 500 patients treated in the UK patient registry. Gut 2015, 64, 1192–1199.
- Hooper, L.V.; Macpherson, A.J. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat. Rev. Immunol. 2010, 10, 159–169.
- Mokili, J.L.; Rohwer, F.; Dutilh, B.E. Metagenomics and future perspectives in virus discovery. Curr. Opin Virol. 2012, 2, 63–77.
- Ursell, L.K.; Haiser, H.J.; Van Treuren, W.; Garg, N.; Reddivari, L.; Vanamala, J.; Knight, R. The intestinal metabolome: An intersection between microbiota and host. Gastroenterology 2014, 146, 1470–1476.
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810.
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270.
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252.
- Wroblewski, L.E.; Peek, R.M.Jr.; Coburn, L.A. The Role of the Microbiome in Gastrointestinal Cancer. Gastroenterol. Clin. North. Am. 2016, 45, 543–556.
- Slattery, M.L.; Benson, J.; Curtin, K.; Ma, K.N.; Schaeffer, D.; Potter, J.D. Carotenoids and colon cancer. Am. J. Clin. Nutr. 2000, 71, 575–582.
- Palozza, P.; Calviello, G.; Serini, S.; Maggiano, N.; Lanza, P.; Ranelletti, F.O.; Bartoli, G.M. Beta-carotene at high concentrations induces apoptosis by enhancing oxy-radical production in human adenocarcinoma cells. Free Radic. Biol. Med. 2001, 30, 1000–1100.
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324.
- Malila, N.; Virtamo, J.; Virtanen, M.; Pietinen, P.; Albanes, D.; Teppo, L. Dietary and serum alpha-tocopherol, beta-carotene and retinol, and risk for colorectal cancer in male smokers. Eur. J. Clin. Nutr. 2002, 56, 615–621.
- Smith-Warner, S.A.; Elmer, P.J.; Tharp, T.M.; Fosdick, L.; Randall, B.; Gross, M.; Potter, J.D. Increasing vegetable and fruit intake: Randomized intervention and monitoring in an at-risk population. Cancer Epidemiol. Biomark. Prev. 2000, 9, 307–317.
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738.
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15.
- Ohnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612.
- Akuffo, K.O.; Nolan, J.; Stack, J.; Moran, R.; Feeney, J.; Kenny, R.A.; Peto, T.; Dooley, C.; O’Halloran, A.M.; Cronin, H. Prevalence of age-related macular degeneration in the Republic of Ireland. Br. J. Ophthalmol. 2015, 99, 1037–1044.
- Ranard, K.M.; Jeon, S.; Mohn, E.S.; Griffiths, J.C.; Johnson, E.J.; Erdman, J.W.J.r. Dietary guidance for lutein: Consideration for intake recommendations is scientifically supported. Eur. J. Nutr. 2017, 56, 37–42.
- Kim, J.; Lee, J.; Oh, J.; Chang, H.J.; Sohn, D.; Kwon, O.; Shin, A.; Kim, J. Dietary Lutein Plus Zeaxanthin Intake and DICER1 rs3742330 A > G Polymorphism Relative to Colorectal Cancer Risk. Sci. Rep. 2019, 9, 3406.
- Collins, A.R.; Harrington, V. Antioxidants; not the only reason to eat fruit and vegetables. Phytochem. Rev. 2003, 1, 167–174.
- Femia, A.P.; Tarquini, E.; Salvadori, M.; Ferri, S.; Giannini, A. K-ras mutations and mucin profile in preneoplastic lesions and colon tumors induced in rats by 1,2-dimethylhydrazine. Int. J. Cancer 2008, 1, 117–123.
- Gali-Muhtasib, H.U.; Younes, I.H.; Karchesy, J.J.; el-Sabban, M.E. Plant tannins inhibit the induction of aberrant crypt foci and colonic tumors by 1,2-dimethylhydrazine in mice. Nutr. Cancer 2001, 39, 108–116.
- Reynoso-Camacho, R.; González-Jasso, E.; Ferriz-Martínez, R.; Villalón-Corona, B.; Salgado, L.; Ramos-Gómez, M. Dietary Supplementation of Lutein Reduces Colon Carcinogenesis in DMH-Treated Rats by Modulating K-ras, PKB, and β-catenin Proteins. Nutr. Cancer 2010, 63, 39–45.
- Satia-Abouta, J.; Galanko, J.A.; Martin, C.F.; Potter, J.D.; Ammerman, A.; Sandler, R.S. Associations of micronutrients with colon cancer risk in African Americans and whites: Results from the North Carolina Colon Cancer Study. Cancer Epidemiol. Biomark. Prev. 2003, 12, 747–754.
- Santocono, M.; Zurria, M.; Berrettini, M.; Fedeli, D.; Falcioni, G. Influence of astaxanthin, zeaxanthin and lutein on DNA damage and repair in UVA-irradiated cells. J. Photochem. Photobiol. B 2006, 85, 205–215.
- Trejo-Solís, C.; Pedraza-Chaverrí, J.; Torres-Ramos, M. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid. Based Complement. Alternat. Med. 2013, 2013, 705121.
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An update on the health effects of tomato lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210.
- Bandeira, A.C.; da Silva, T.P.; de Araujo, G.R. Lycopene inhibits reactive oxygen species production in SK-Hep-1 cells and attenuates acetaminophen-induced liver injury in C57BL/6 mice. Chem. Biol. Interact. 2016, 263, 7–17.
- Boehm, F.; Edge, R.; Truscott, T.G.; Witt, C. A dramatic effect of oxygen on protection of human cells against γ-radiation by lycopene. FEBS Lett. 2016, 590, 1086–1093.
- Slattery, M.L.; Lundgreen, A.; Welbourn, B.; Wollf, R.K.; Corcoran, C. Oxidative balance and colon and rectal cancer: Interaction of lifestyle factors and genes. Mutat. Res. 2012, 734, 30–40.
- Youn, S.W. Systemic inflammatory response as a prognostic factor in patients with cancer. J. Korean Orient Oncol. 2012, 17, 1–7.
- Lin, M.C.; Wang, F.Y.; Kuo, Y.H.; Tang, F.Y. Cancer chemopreventive effects of lycopene: Suppression of MMP-7 expression and cell invasion in human colon cancer cells. J. Agric. Food Chem. 2011, 59, 11304–11318.
- Palozza, P.; Colangelo, M.; Simone, R. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis 2010, 31, 1813–1821.
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90–96.
- Bhuvaneswari, V.; Velmurugan, B.; Nagini, S. Lycopene, an antioxidant carotenoid modulates glutathione-dependent hepatic biotransformation enzymes during experimental gastric carcinogenesis. Nutr. Res. 2001, 8, 1117–1124.
- De la Iglesia, R.; Milagro, F.I.; Campion, J.; Boque, N.; Martinez, J.A. Healthy properties of proanthocyanidins. Biofactors 2010, 36, 159–168.
- Blade, C.; Aragones, G.; Arola-Arnal, A.; Muguerza, B.; Bravo, F.I.; Salvado, M.J.; Suarez, M. Proanthocyanidins in health and disease. Biofactors 2016, 42, 5–12.
- Cos, P.; De Bruyne, T.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2004, 11, 1345–1359.
- Casanova-Marti, A.; Serrano, J.; Portune, K.J.; Sanz, Y.; Blay, M.T.; Terra, X.; Pinent, M. Grape seed proanthocyanidins influence gut microbiota and enteroendocrine secretions in female rats. Food Funct. 2018, 9, 1672–1682.
- Lee, Y. Cancer Chemopreventive Potential of Procyanidin. Toxicol. Res. 2017, 33, 273–282.
- Neto, C.C. Cranberries: Ripe for more cancer research? J. Sci. Food Agric. 2011, 91, 2303–2307.
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.F.; Lacroix, M. Bioactive compounds in cranberries and their biological properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 666–679.
- Pappas, E.; Schaich, K.M. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2009, 49, 741–781.
- Duthie, S.J.; Jenkinson, A.M.; Crozier, A.; Mullen, W.; Pirie, L.; Kyle, J.; Yap, L.S.; Christen, P.; Duthie, G.G. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur. J. Nutr. 2006, 45, 113–122.
- Wu, X.; Song, M.; Cai, X.; Neto, C.; Tata, A.; Han, Y.; Xiao, H. Chemopreventive Effects of Whole Cranberry (Vaccinium macrocarpon) on Colitis-Associated Colon Tumorigenesis. Mol. Nutr. Food Res. 2018, 62, e1800942.
- Boateng, J.; Verghese, M.; Shackelford, L.; Walker, L.T.; Khatiwada, J.; Ogutu, S.; Chawan, C.B. Selected fruits reduce azoxymethane (AOM)-induced aberrant crypt foci (ACF) in Fisher 344 male rats. Food Chem. Toxicol. 2007, 45, 725–732.
- Xiao, S.D.; Shi, T. Is cranberry juice effective in the treatment and prevention of Helicobacter pyloriinfection of mice? Chin. J. Dig. Dis. 2003, 4, 136–139.
- Jin, D.; Liu, T.; Dong, W.; Zhang, Y.; Wang, S.; Xie, R.; Cao, H. Dietary feeding of freeze-dried whole cranberry inhibits intestinal tumor development in Apc(min/+) mice. Oncotarget 2017, 8, 97787–97800.
- Koosha, S.; Alshawsh, M.A.; Looi, C.Y.; Seyedan, A.; Mohamed, Z. An Association Map on the Effect of Flavonoids on the Signaling Pathways in Colorectal Cancer. Int. J. Med. Sci. 2016, 13, 374–385.
- Sun, Q.; Yue, Y.; Shen, P.; Yang, J.J.; Park, Y. Cranberry Product Decreases Fat Accumulation in Caenorhabditis elegans. J. Med. Food. 2016, 19, 427–433.
- Upton, R. Bilberry Fruit Vaccinium myrtillus L. Standards of Analysis, Quality Control, and Therapeutics; AHP: Santa Cruz, CA, USA, 2001.
- Chu, W.; Cheung, S.C.M.; Lau, R.A.W.; Benzie, I.F.F. Bilberry (Vaccinium myrtillus L.). In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, Sissi, Eds.; CRC Press: Boca Raton, FL, USA, 2011.
- Mazza, G.; Kay, C.D.; Correll, T.; Holub, B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002, 50, 7731–7737.
- Valentova, K.; Ulrichova, J.; Cvak, L.; Simanek, V. Cytoprotective effect of a bilberry extract against oxidative damage of rat hepatocytes. Food Chem. 2006, 101, 912–917.
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 760689.
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-rich extracts inhibit multiple biomarkers of colon cancer in rats. Nutr. Cancer 2006, 54, 84–93.
- Mutanen, M.; Pajari, A.M.; Paivarinta, E.; Misikangas, M.; Rajakangas, J.; Marttinen, M.; Oikarinen, S. Berries as preventive dietary constituents-a mechanistic approach with ApcMin+ mouse. Asia Pac. J. Clin. Nutr. 2008, 17, 123–125.
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290.
- Lippert, E.; Ruemmele, P.; Obermeier, F.; Goelder, S.; Kunst, C.; Rogler, G.; Endlicher, E. Anthocyanins Prevent Colorectal Cancer Development in a Mouse Model. Digestion 2017, 95, 275–280.
- Thomasset, S.; Berry, D.P.; Cai, H.; West, K.; Marczylo, T.H.; Marsden, D.; Gescher, A.J. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer Prev. Res. Phila 2009, 2, 625–633.
- Esselen, M.; Fritz, J.; Hutter, M.; Teller, N.; Baechler, S.; Boettler, U.; Marko, D. Anthocyanin-rich extracts suppress the DNA-damaging effects of topoisomerase poisons in human colon cancer cells. Mol. Nutr. Food Res. 2011, 55, S143–S153.
- Chau, I.; Cunningham, D. Adjuvant therapy in colon cancer—what, when and how? Ann. Oncol. 2006, 17, 1347–1359.
- Xiao, D.; Pinto, J.T.; Gundersen, G.G.; Weinstein, I.B. Effects of a series of organosulfur compounds on mitotic arrest and induction of apoptosis in colon cancer cells. Mol. Cancer Ther. 2005, 4, 1388–1398.
- Moriarty, R.M.; Naithani, R.; Surve, B. Organosulfur compounds in cancer chemoprevention. Mini. Rev. Med. Chem. 2007, 7, 827–838.
- Nagini, S. Cancer chemoprevention by garlic and its organosulfur compounds-panacea or promise? Anticancer Agents Med. Chem. 2008, 8, 313–321.
- El-Bayoumy, K.; Sinha, R.; Pinto, J.T. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J. Nutr. 2016, 136, S864–S869.
- Hu, J.Y.; Hu, Y.W.; Zhou, J.J.; Zhang, M.W.; Li, D.; Zheng, S. Consumption of garlic and risk of colorectal cancer: An updated meta-analysis of prospective studies. World J. Gastroenterol. 2014, 20, 15413–15422.
- Ross, S.A.; Finley, J.W.; Milner, J.A. Allyl sulfur compounds from garlic modulate aberrant crypt formation. J. Nutr. 2006, 136, S852–S854.
- Powolny, A.A.; Singh, S.V. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related allium vegetable-derived organosulfur compounds. Cancer Lett. 2008, 269, 305–314.
- Yi, L.; Su, Q. Molecular mechanisms for the anticancer effects of diallyl disulfide. Food Chem. Toxicol. 2013, 57, 362–370.
- Roy, N.; Nazeem, P.A.; Babu, T.D.; Abida, P.S.; Narayanankutty, A.; Valsalan, R.; Raghavamenon, A.C. EGFR gene regulation in colorectal cancer cells by garlic phytocompounds with special emphasis on S-Allyl-L-Cysteine Sulfoxide. Interdiscip Sci. 2008, 10, 686–693.
- Saud, S.M.; Li, W.; Gray, Z.; Matter, M.S.; Colburn, N.H.; Young, M.R.; Kim, Y.S. Diallyl Disulfide (DADS), a Constituent of Garlic, Inactivates NF-kappaB and Prevents Colitis-Induced Colorectal Cancer by Inhibiting GSK-3beta. Cancer Prev. Res. Phila 2016, 9, 607–615.
- Janakiram, N.B.; Rao, C.V. The role of inflammation in colon cancer. Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2014; Volume 816, pp. 25–52.
- Erstad, D.J.; Cusack, J.C. Targeting the NF-kappaB pathway in cancer therapy. Surg. Oncol. Clin. N. Am. 2013, 22, 705–746.
- Li, S.; Yang, G.; Zhu, X.; Cheng, L.; Sun, Y.; Zhao, Z. Combination of rapamycin and garlic-derived S-allylmercaptocysteine induces colon cancer cell apoptosis and suppresses tumor growth in xenograft nude mice through autophagy/p62/Nrf2 pathway. Oncol. Rep. 2017, 38, 1637–1644.
- Raghu, R.; Lu, K.H.; Sheen, L.Y. Recent Research Progress on Garlic (da suan) as a Potential Anticarcinogenic Agent Against Major Digestive Cancers. J. Tradit. Complement Med. 2012, 2, 192–201.
- Zhou, Y.; Zhuang, W.; Hu, W.; Liu, G.J.; Wu, T.X.; Wu, X.T. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology 2011, 141, 80–89.
- Griffiths, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions--a global benefit to health. Phytother. Res. 2002, 16, 603–615.
- Suleria, H.A.; Butt, M.S.; Anjum, F.M.; Saeed, F.; Khalid, N. Onion: Nature protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2015, 55, 50–66.
- Izzo, A.A.; Capasso, R.; Capasso, F. Eating garlic and onion: A matter of life or death. Br. J. Cancer 2004, 91, 194.
- Murayyan, A.I.; Manohar, C.M.; Hayward, G.; Neethirajan, S. Antiproliferative activity of Ontario grown onions against colorectal adenocarcinoma cells. Food Res. Int. 2017, 96, 12–18.
- He, Y.; Jin, H.; Gong, W.; Zhang, C.; Zhou, A. Effect of onion flavonoids on colorectal cancer with hyperlipidemia: An in vivo study. Onco Targets Ther. 2014, 7, 101–110.
- Tung, Y.C.; Tsai, M.L.; Kuo, F.L.; Lai, C.S.; Badmaev, V.; Ho, C.T.; Pan, M.H. Se-Methyl-L-selenocysteine Induces Apoptosis via Endoplasmic Reticulum Stress and the Death Receptor Pathway in Human Colon Adenocarcinoma COLO 205 Cells. J. Agric. Food Chem. 2015, 63, 5008–5016.
- Ibanez-Redin, G.; Furuta, R.H.M.; Wilson, D.; Shimizu, F.M.; Materon, E.M.; Arantes, L.; Oliveira, O.N. Screen-printed interdigitated electrodes modified with nanostructured carbon nano-onion films for detecting the cancer biomarker CA19-9. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1502–1508.
- Negi, J.S.; Singh, P.; Joshi, G.P.; Rawat, M.S.; Bisht, V.K. Chemical constituents of Asparagus. Pharm. Rev. 2010, 4, 215–220.
- Saxena, V.K.; Chaurasia, S. A new isoflavone from the roots of Asparagus racemosus. Fitoterapia 2001, 72, 307–309.
- Hamdi, A.; Jaramillo-Carmona, S.; Srairi Beji, R.; Tej, R.; Zaoui, S.; Rodriguez-Arcos, R.; Guillen-Bejarano, R. The phytochemical and bioactivity profiles of wild Asparagus albus L. plant. Food Res. Int. 2017, 99, 720–729.
- Jaramillo-Carmona, S.; Guillen-Bejarano, R.; Jimenez-Araujo, A.; Rodriguez-Arcos, R.; Lopez, S. In Vitro Toxicity of Asparagus Saponins in Distinct Multidrug-Resistant Colon Cancer Cells. Chem. Biodivers. 2018, 15, e1800282.
- Zhang, W.; He, W.; Shi, X.; Li, X.; Wang, Y.; Hu, M.; Qin, Z. An Asparagus polysaccharide fraction inhibits MDSCs by inducing apoptosis through toll-like receptor 4. Phytother. Res. 2018, 32, 1297–1303.
- Wang, J.; Liu, Y.; Zhao, J.; Zhang, W.; Pang, X. Saponins extracted from by-product of Asparagus officinalis L. suppress tumour cell migration and invasion through targeting Rho GTPase signalling pathway. J. Sci. Food Agric. 2013, 93, 1492–1498.
- Bousserouel, S.; Le Grandois, J.; Gosse, F.; Werner, D.; Barth, S.W.; Marchioni, E.; Raul, F. Methanolic extract of white asparagus shoots activates TRAIL apoptotic death pathway in human cancer cells and inhibits colon carcinogenesis in a preclinical model. Int. J. Oncol. 2013, 43, 394–404.
- Tse, G.; Eslick, G.D. Cruciferous vegetables and risk of colorectal neoplasms: A systematic review and meta-analysis. Nutr. Cancer 2014, 66, 128–139.
- Burow, M.; Bergner, A.; Gershenzon, J.; Wittstock, U. Glucosinolate hydrolysis in Lepidium sativum—Identification of the thiocyanate-forming protein. Plant Mol. Biol. 2007, 63, 49–61.
- Koroleva, O.A.; Davies, A.; Deeken, R.; Thorpe, M.R.; Tomos, A.D.; Hedrich, R. Identification of a New Glucosinolate-Rich Cell Type in Arabidopsis Flower Stalk. Plant Physiol. 2000, 124, 599–608.
- Clarke, J.D.; Dashwood, R.H.; Ho, E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008, 269, 291–304.
- Ramos-Gomez, M.; Kwak, M.K.; Dolan, P.M.; Itoh, K.; Yamamoto, M.; Talalay, P.; Kensler, T.W. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. USA 2001, 98, 3410–3415.
- Gupta, P.; Kim, B.; Kim, S.H.; Srivastava, S.K. Molecular targets of isothiocyanates in cancer: Recent advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707.
- Higgins, L.G.; Kelleher, M.O.; Eggleston, I.M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox–cycling agents. Toxicol. Appl. Pharmacol. 2009, 237, 267–280.
- Keum, Y.S.; Yu, S.; Chang, P.P.; Yuan, X.; Kim, J.H.; Xu, C.; Han, J.; Agarwal, A.; Kong, A.N. Mechanism of Action of Sulforaphane: Inhibition of p38 Mitogen-Activated Protein Kinase Isoforms Contributing to the Induction of Antioxidant Response Element-Mediated Heme Oxygenase-1 in Human Hepatoma HepG2 Cells. Cancer Res. 2006, 66, 8804–8813.
- Kim, J.K.; Gallaher, D.D.; Chen, C.; Gallaher, C.M.; Yao, D.; Trudo, S.P. Phenethyl isothiocyanate and indole-3-carbinol from cruciferous vegetables, but not furanocoumarins from apiaceous vegetables, reduced PhIP-induced DNA adducts in Wistar rats. Mol. Nutr. Food Res. 2016, 60, 1956–1966.
- Byun, S.; Shin, S.H.; Park, J.; Lim, S.; Lee, E.; Lee, C.; Sung, D.; Farrand, L.; Lee, S.R.; Kim, K.H.; et al. Sulforaphene suppresses growth of colon cancer-derived tumors via induction of glutathione depletion and microtubule depolymerization. Mol. Nutr. Food Res. 2016, 60, 1068–1078.
- Choi, H.J.; Lim, D.Y.; Park, J.H. Induction of G1 and G2/M cell cycle arrests by the dietary compound 3,30-diindolylmethane in HT-29 human colon cancer cells. BMC Gastroenterol. 2009, 9, 39.
- Johnson, I.T. Cruciferous Vegetables and Risk of Cancers of the Gastrointestinal Tract. Mol. Nutr. Food Res. 2018, 62, e1701000.
- Shebaby, W.N.; Bodman-Smith, K.B.; Mansour, A.; Mroueh, M.; Taleb, R.I.; El-Sibai, M.; Daher, C.F. Daucus carota Pentane-Based Fractions Suppress Proliferation and Induce Apoptosis in Human Colon Adenocarcinoma HT-29 Cells by Inhibiting the MAPK and PI3K Pathways. J. Med. Food. 2005, 18, 745–752.
- Purup, S.; Larsen, E.; Christensen, L.P. Differential effects of falcarinol and related aliphatic C-polyacetylenes on intestinal cell proliferation. J. Agric. Food Chem. 2009, 57, 8290–8296.
- Pan, M.H.; Ho, C.T. Chemopreventive effects of natural dietary compounds on cancer development. Chem. Soc. Rev. 2008, 37, 2558–2574.
- Sriamornsak, P. Chemistry of pectin and its pharmaceutical uses: A Review. Silpakorn Univ. Int. J. 2003, 3, 206–228.
- Ciriminna, R.; Fidalgo, A.; Delisi, R.; Tamburino, A.; Carnaroglio, D.; Cravotto, G.; Pagliaro, M. Controlling the Degree of Esterification of Citrus Pectin for Demanding Applications by Selection of the Source. ACS Omega 2017, 2, 7991–7995.
- Jalili-Nik, M.; Soltani, A.; Moussavi, S.; Ghayour-Mobarhan, M.; Ferns, G.A.; Hassanian, S.M.; Avan, A. Current status and future prospective of Curcumin as a potential therapeutic agent in the treatment of colorectal cancer. J. Cell. Physiol. 2018, 233, 6337–6345.
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis., N.H.; Naidu, R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2454.
- He, S.; Simpson, B.K.; Sun, H.; Ngadi, M.O.; Ma, Y.; Huang, T. Phaseolus vulgaris lectins: A systematic review of characteristics and health implications. Crit. Rev. Food Sci. Nutr. 2018, 58, 70–83.
- Hangen, L.; Bennink, M.R. Consumption of black beans and navy beans (Phaseolus vulgaris) reduced azoxymethane-induced colon cancer in rats. Nutr. Cancer 2002, 44, 60–65.
- Law, B.M.H.; Waye, M.M.Y.; So, W.K.W.; Chair, S.Y. Hypotheses on the Potential of Rice Bran Intake to Prevent Gastrointestinal Cancer through the Modulation of Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 1352.
- Ryan, E.P.; Heuberger, A.L.; Weir, T.L.; Barnett, B.; Broeckling, C.D.; Prenni, J.E. Rice bran fermented with Saccharomyces boulardii generates novel metabolite profiles with bioactivity. J. Agric. Food Chem. 2011, 59, 1862–1870.




