
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Manon Owen | + 2568 word(s) | 2568 | 2021-06-03 09:47:14 | | | |
| 2 | Rita Xu | -25 word(s) | 2543 | 2021-06-07 06:32:42 | | |
Video Upload Options
Metformin is a first-line treatment for many people with type 2 diabetes mellitus (T2DM) and gestational diabetes mellitus (GDM) to maintain glycaemic control. Although metformin demonstrates beneficial and protective cardiovascular effects for the mother, evidence suggests metformin may not be favourable for the short and long-term metabolic health of the offspring. Metformin can cross the placenta and could have a role in fetal programming.
1. Introduction
2. Metformin in Pregnancy
| Reference | Model | Effects Demonstrated by Metformin |
Significance |
|---|---|---|---|
| Clinical studies | |||
| Jamal et al. 2012 [32] |
Pregnant women with PCOS treated with metformin | - ⇔ on birth weight - ↓ uterine artery pulsatility index |
Metformin adversely affected uteroplacental circulation |
| Ex vivo or in vitro human placental studies | |||
| Jiang et al. 2020 [33] |
Human GDM and T2DM placental explants cultured and treated with metformin (ex vivo) | Male human placental explants: - AMPK activation - ↑ H3K27 acetylation - ↓ DNMT1 protein abundance - ↓ PGC-1α promoter methylation and ↑ PGC-1α mRNA expression |
Effects of metformin may be fetal sex-dependent Metformin may improve placental efficiency by facilitating placental mitochondrial biogenesis |
| Brownfoot et al. 2020 [34] Cluver et al. 2019 [35] Kaitu’u-Lino 2018 [36] Brownfoot et al. 2016 [37] |
Human primary tissues exposed to metformin; placental explants, endothelial cells and placental villous explants, whole maternal vessels, maternal omental vessel explants (in vitro and ex vivo) | - ↓ sFlt-1 and sEng secretion from primary endothelial cells, preterm preeclamptic placental villous explants and villous cytotrophoblast cells - ↓ VCAM-1 mRNA expression in endothelial cells - ↑ whole maternal blood vessel angiogenesis - ↓ sFlt mRNA expression - ↓ TNFα-mediated endothelial cell dysfunction |
Metformin enhances placental angiogenesis and reduces endothelial dysfunction by decreasing endothelial and trophoblastic antiangiogenic factor secretion via mitochondrial electron transport chain inhibition Metformin is being trialled as a medication for preeclampsia (trial number PACTR201608001752102) |
| Szukiewicz et al. 2018 [38] |
Human placental lobules perfused with metformin under normoglycemic or hyperglycaemic conditions (ex vivo) |
- ↓ CX3CL1 and TNFα secretion - ↑ placental CX3CR1 protein expression - ↓ placental NFκB p65 protein |
Metformin has anti-inflammatory effects in the placenta |
| Correia-Branco et al. 2018 [39] |
HTR-8/SVneo extravillous trophoblast cell line exposed to metformin (in vitro) |
- ↓ proliferation - ↑ apoptosis - Inhibited folic acid uptake - Inhibited glucose uptake - Effects of metformin were prevented by inhibition of mTOR, JNK, and PI3K pathways |
Metformin impairs placental development and nutrient transport via PI3K, mTOR, JNK, and PI3K pathways |
| Arshad et al. 2016 [40] |
Human placental explants; from healthy pregnancy, non-treated diet-controlled GDM pregnancy, and metformin-treated GDM pregnancy (ex vivo) | - ↓ similar morphology in metformin-treated GDM placenta and non-treated healthy placenta, except for increased cord width - ↓ placental width in metformin-treated GDM placenta compared to non-treated GDM placenta - ↓ chorangiosis, placental thickness, and syncytial knots in metformin-treated placenta compared to non-treated GDM placenta |
Metformin may improve placental morphology by restoring diabetic placental hallmarks to characteristics similar to healthy placenta |
| Han et al. 2015 [41] |
Human first trimester trophoblasts treated with or without metformin (in vitro) | - ↓ trophoblast cytokine and chemokine release in normal and high glucose culture concentrations - No antiangiogenic or antimigratory effects |
Metformin may potentially decrease placental glucose-induced inflammatory response |
| In vivo rodent studies | |||
| Jiang et al. 2020 [33] |
Mice treated with maternal metformin and high-fat diet | Improved placental efficiency in males: - ↓ PGC-1α promoter methylation and ↑ PGC-1α expression - ↑ TFAM expression Improved glucose homeostasis in male offspring |
Metformin may improve placental efficiency by facilitating placental mitochondrial biogenesis Metformin may be protective to the offspring by suppressing epigenetic changes evoked by maternal diabetes |
| Wang et al. 2019 [42] |
Pregnant mice fed an isocaloric diet (control), high-fat diet, or high-fat diet plus metformin (in vivo) |
- ↓ placental weight compared to control - Partially rescued high-fat diet induced ↓ in placental and fetal weight - ↑ VEGF and MMP-2 protein expression |
Metformin improves high fat diet-induced reduction in placental and fetal growth, potentially by modulating placental vasculature |
| Alzamendi et al. 2012 [43] |
Pregnant rats fed a normal or high-fructose diet, treated with metformin (in vivo) |
- ↓ fetal weight - ⇔ on placental weight or blood vessel area - Improved fructose diet induced ↓ blood vessel area |
Metformin reduces fetal weight in mice fed a normal diet Metformin prevents high fructose diet-induced placental dysfunction |
Dark grey is table heading; pale grey titles demonstrate whether the study was clinical, ex-vivo or in vitro human placental, or in-vivo rodent studies. ⇔ no change; ↓reduction; ↑ increase. AMPK, AMP-activated protein kinase; DNMT, DNA methyltransferase; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator 1α; TFAM, mitochondrial transcription factor A; sFlt-1, soluble fms-like tyrosine kinase-1; sEng, soluble endoglin; VCAM-1, vascular cell adhesion molecule 1; TNFα, tumour necrosis factor alpha; VEGF, vascular endothelial growth factor; MMP-2, matrix metalloproteinase-2; NF-κB, nuclear factor kappa B; mTOR, mammalian target of rapamycin; JNK, c-Jun N-terminal kinase; PI3K, hosphatidylinositol-3-kinase.
2.1. Transplacental Transport of Metformin
2.2. Impact of Metformin on Placental Nutrient Transport and Nutrient Bioavailabilty
References
- International Diabetes Federation (IDF). World Diabetes Day 2017 to Focus on Women and Diabetes, Belgium. 2017. Available online: (accessed on 10 December 2020).
- Melchior, H.; Kurch-Bek, D.; Mund, M. The prevalence of gestational diabetes. Dtsch. Arztebl. Int. 2017, 114, 412–418.
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019.
- Zhu, Y.; Zhang, C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: A global perspective. Curr. Diab. Rep. 2016, 16, 7.
- Tarry-Adkins, J.L.; Aiken, C.E.; Ozanne, S.E. Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: A systematic review and meta-analysis. PLOS Med. 2019, 16, e1002848.
- Stacey, T.; Tennant, P.; McCowan, L.; Mitchell, E.; Budd, J.; Li, M.; Thompson, J.; Martin, B.; Roberts, D.; Heazell, A. Gestational diabetes and the risk of late stillbirth: A case–control study from England, UK. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 973–982.
- Feig, D.S.; Donovan, L.E.; Zinman, B.; Sanchez, J.J.; Asztalos, E.; Ryan, E.A.; Fantus, I.G.; Hutton, E.; Armson, A.B.; Lipscombe, L.L.; et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): A multicentre, international, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 834–844.
- Lee, A.J.; Hiscock, R.J.; Wein, P.; Walker, S.P.; Permezel, M. Gestational diabetes mellitus: Clinical predictors and long-term risk of developing type 2 diabetes. A retrospective cohort study using survival analysis. Diabetes Care 2007, 30, 878–883.
- Engeland, A.; Bjørge, T.; Daltveit, A.K.; Skurtveit, S.; Vangen, S.; Vollset, S.E.; Furu, K. Risk of diabetes after gestational diabetes and preeclampsia. A registry-based study of 230,000 women in Norway. Eur. J. Epidemiol. 2011, 26, 157–163.
- Saravanan, P. Gestational diabetes: Opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020, 8, 793–800.
- Whicher, C.A.; O’Neill, S.; Holt, R.I.G. Diabetes in the UK: 2019. Diabet. Med. 2020, 37, 242–247.
- Murphy, H.R.; Howgate, C.; O’Keefe, J.; Myers, J.; Morgan, M.; Coleman, M.A.; Jolly, M.; Valabhji, J.; Scott, E.M.; Knighton, P.; et al. Characteristics and outcomes of pregnant women with type 1 or type 2 diabetes: A 5-year national population-based cohort study. Lancet Diabetes Endocrinol. 2021, 9, 153–164.
- Kelley, K.W.; Carroll, D.G.; Meyer, A. A review of current treatment strategies for gestational diabetes mellitus. Drugs Context 2015, 4.
- Bahendeka, S.; Kaushik, R.; Swai, A.B.; Otieno, F.; Bajaj, S.; Kalra, S.; Bavuma, C.M.; Karigire, C. EADSG guidelines: Insulin storage and optimisation of injection technique in diabetes management. Diabetes Therapy. 2019, 10, 341–366.
- Wakeman, M.; Archer, D.T. Metformin and micronutrient status in type 2 diabetes: Does polypharmacy involving acid-suppressing medications affect vitamin B12 levels? Diabetes Metab. Syndr. Obes. 2020, 13, 2093–2108.
- Lindsay, R.S.; Loeken, M.R. Metformin use in pregnancy: Promises and uncertainties. Diabetologia 2017, 60, 1612–1619.
- Bridges, H.R.; Jones, A.J.Y.; Pollak, M.N.; Hirst, J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 2014, 462, 475–487.
- Yang, L.; Garcia Canaveras, J.C.; Chen, Z.; Wang, L.; Liang, L.; Jang, C.; Mayr, J.A.; Zhang, Z.; Ghergurovich, J.M.; Zhan, L.; et al. Serine catabolism feeds NADH when respiration is impaired. Cell Metab. 2020, 31, 809–821.
- Lee, N.; Hebert, M.F.; Wagner, D.J.; Easterling, T.R.; Liang, C.J.; Rice, K.; Wang, J. Organic cation transporter 3 facilitates fetal Exposure to metformin during pregnancy. Mol. Pharmacol. 2018, 94, 1125–1131.
- Cuyàs, E.; Fernández-Arroyo, S.; Buxó, M.; Pernas, S.; Dorca, J.; Álvarez, I.; Martínez, S.; Pérez-Garcia, J.M.; Batista-López, N.; Rodríguez-Sánchez, C.A.; et al. Metformin induces a fasting- and antifolate-mimicking modification of systemic host metabolism in breast cancer patients. Aging 2019, 11, 2874–2888.
- Barbour, L.A.; Scifres, C.; Valent, A.M.; Friedman, J.E.; Buchanan, T.A.; Coustan, D.; Aagaard, K.; Thornburg, K.L.; Catalano, P.M.; Galan, H.L.; et al. A cautionary response to SMFM statement: Pharmacological treatment of gestational diabetes. Am. J. Obstet. Gynecol. 2018, 219.
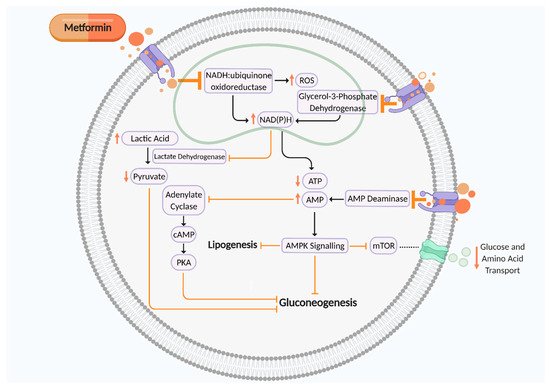
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; MacDonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 2014, 510, 542–546.
- An, H.; He, L. Current understanding of metformin effect on the control of hyperglycemia in diabetes. J. Endocrinol. 2016, 228.
- Hyer, S.; Balani, J.; Shehata, H. Metformin in pregnancy: Mechanisms and clinical applications. Int. J. Mol. Sci. 2018, 19, 1954.
- Balani, J.; Hyer, S.L.; Rodin, D.A.; Shehata, H. Pregnancy outcomes in women with gestational diabetes treated with metformin or insulin: A case-control study. Diabet. Med. 2009, 26, 798–802.
- Lee, P.A.; Chernausek, S.D.; Hokken-Koelega, A.C.S.; Czernichow, P. International small for gestational age advisory board consensus development conference statement: Management of short children born small for gestational age, april 24–october 1, 2001. Pediatrics 2003, 111, 1253–1261.
- Rowan, J.A.; Rush, E.C.; Obolonkin, V.; Battin, M.; Wouldes, T.; Hague, W.M. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): Body composition at 2 years of age. Diabetes Care 2011, 34, 2279–2284.
- Rowan, J.A.; Rush, E.C.; Plank, L.D.; Lu, J.; Obolonkin, V.; Coat, S.; Hague, W.M. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): Body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res. Care 2018, 6.
- Hanem, L.G.E.; Salvesen, Ø.; Juliusson, P.B.; Carlsen, S.M.; Nossum, M.C.F.; Vaage, M.; Ødegård, R.; Vanky, E. Intrauterine metformin exposure and offspring cardiometabolic risk factors (PedMet study): A 5–10 year follow-up of the PregMet randomised controlled trial. Lancet Child. Adolesc. Health 2019, 3, 166–174.
- Ijäs, H.; Vääräsmäki, M.; Saarela, T.; Keravuo, R.; Raudaskoski, T. A follow-up of a randomised study of metformin and insulin in gestational diabetes mellitus: Growth and development of the children at the age of 18 months. BJOG 2015, 122, 994–1000.
- Salomäki, H.; Vähätalo, L.H.; Laurila, K.; Jäppinen, N.T.; Penttinen, A.-M.; Ailanen, L.; Ilyasizadeh, J.; Pesonen, U.; Koulu, M. Prenatal metformin exposure in mice programs the metabolic phenotype of the offspring during a high fat diet at adulthood. PLoS ONE 2013, 8, e56594.
- Jamal, A.; Milani, F.; Al-Yasin, A. Evaluation of the effect of metformin and aspirin on utero placental circulation of pregnant women with PCOS. Iran. J. Reprod. Med. 2012, 10, 265–270.
- Jiang, S.; Teague, A.M.; Tryggestad, J.B.; Jensen, M.E.; Chernausek, S.D. Role of metformin in epigenetic regulation of placental mitochondrial biogenesis in maternal diabetes. Sci. Rep. 2020, 10.
- Brownfoot, F.C.; Hastie, R.; Hannan, N.J.; Cannon, P.; Nguyen, T.V.; Tuohey, L.; Cluver, C.; Tong, S.; Kaitu’u-Lino, T.J. Combining metformin and sulfasalazine additively reduces the secretion of antiangiogenic factors from the placenta: Implications for the treatment of preeclampsia. Placenta 2020, 95, 78–83.
- Cluver, C.; Walker, S.P.; Mol, B.W.; Hall, D.; Hiscock, R.; Brownfoot, F.C.; Kaitu’u-Lino, T.J.; Tong, S. A double blind, randomised, placebo-controlled trial to evaluate the efficacy of metformin to treat preterm pre-eclampsia (PI2 Trial): Study protocol. BMJ Open 2019, 9, e025809.
- Kaitu’u-Lino, T.J.; Brownfoot, F.C.; Beard, S.; Cannon, P.; Hastie, R.; Nguyen, T.V.; Binder, N.K.; Tong, S.; Hannan, N.J. Combining metformin and esomeprazole is additive in reducing sFlt-1 secretion and decreasing endothelial dysfunction—Implications for treating preeclampsia. PLoS ONE 2018, 13, e0188845.
- Brownfoot, F.C.; Hastie, R.; Hannan, N.J.; Cannon, P.; Tuohey, L.; Parry, L.J.; Senadheera, S.; Illanes, S.E.; Kaitu’u-Lino, T.J.; Tong, S. Metformin as a prevention and treatment for preeclampsia: Effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am. J. Obstet. Gynecol. 2016, 214.
- Szukiewicz, D.; Szewczyk, G.; Pyzlak, M.; Stangret, A.; Bachanek, M.; Trojanowski, S.; Alkhalayla, H.; Wejman, J. Anti-inflammatory action of metformin with respect to CX3CL1/CX3CR1 signaling in human placental circulation in normal-glucose versus high-glucose environments. Inflammation 2018, 41, 2246–2264.
- Correia-Branco, A.; Keating, E.; Martel, F. Involvement of mTOR, JNK and PI3K in the negative effect of ethanol and metformin on the human first-trimester extravillous trophoblast HTR-8/SVneo cell line. Eur. J. Pharmacol. 2018, 833, 16–24.
- Arshad, R.; Kanpurwala, M.A.; Karim, N.; Hassan, J.A. Effects of diet and metformin on placental morphology in gestational diabetes mellitus. Pak. J. Med. Sci. 2016, 32, 1522–1527.
- Han, C.S.; Herrin, M.A.; Pitruzzello, M.C.; Mulla, M.J.; Werner, E.F.; Pettker, C.M.; Flannery, C.A.; Abrahams, V.M. Glucose and metformin modulate human first trimester trophoblast function: A model and potential therapy for diabetes-associated uteroplacental insufficiency. Am. J. Reprod. Immunol. 2014, 73, 362–371.
- Wang, F.; Cao, G.; Yi, W.; Li, L.; Cao, X. Effect of metformin on a preeclampsia-like mouse model induced by high-fat diet. BioMed Res. Int. 2019, 2019.
- Alzamendi, A.; Del Zotto, H.; Castrogiovanni, D.; Romero, J.; Giovambattista, A.; Spinedi, E. Oral metformin treatment prevents enhanced insulin demand and placental dysfunction in the pregnant rat fed a fructose-rich diet. ISRN Endocrinol. 2012, 2012.
- Eyal, S.; Easterling, T.R.; Carr, D.; Umans, J.G.; Miodovnik, M.; Hankins, G.D.V.; Clark, S.M.; Risler, L.; Wang, J.; Kelly, E.J.; et al. Pharmacokinetics of metformin during pregnancy. Drug Metab. Dispos. 2010, 38, 833–840.
- Charles, B.; Norris, R.; Xiao, X.; Hague, W. Population pharmacokinetics of metformin in late pregnancy. Ther. Drug Monit. 2006, 28, 67–72.
- Vanky, E.; Zahlsen, K.; Spigset, O.; Carlsen, S.M. Placental passage of metformin in women with polycystic ovary syndrome. Fertil. Steril. 2005, 83, 1575–1578.
- Liao, M.Z.; Flood Nichols, S.K.; Ahmed, M.; Clark, S.; Hankins, G.D.; Caritis, S.; Venkataramanan, R.; Haas, D.; Quinney, S.K.; Haneline, L.S.; et al. Effects of pregnancy on the pharmacokinetics of metformin. Drug Metab. Dispos. 2020, 48, 264–271.
- Gormsen, L.C.; Sundelin, E.I.; Jensen, J.B.; Vendelbo, M.H.; Jakobsen, S.; Munk, O.L.; Hougaard Christensen, M.M.; Brøsen, K.; Frøkiær, J.; Jessen, N. In vivo Imaging of human 11C-metformin in peripheral organs: Dosimetry, biodistribution, and kinetic analyses. J. Nucl. Med. 2016, 57, 1920–1926.
- Ganapathy, V.; Prasad, P.D. Role of transporters in placental transfer of drugs. Toxicol. Appl. Pharmacol. 2005, 207, 381–387.
- Han, T.; Proctor, W.R.; Costales, C.L.; Cai, H.; Everett, R.S.; Thakker, D.R. Four cation-selective transporters contribute to apical uptake and accumulation of metformin in caco-2 cell monolayers. J. Pharmacol. Exp. Ther. 2015, 352, 519–528.
- Grube, M.; Meyer, Z.; Schwabedissen, H.; Draber, K.; Präger, D.; Möritz, K.U.; Linnemann, K.; Fusch, C.; Jedlitschky, G.; Kroemer, H.K. Expression, localization, and function of the carnitine transporter octn2 (slc22a5) in human placenta. Drug Metab. Dispos. 2005, 33, 31–37.
- Lee, N.; Hebert, M.F.; Prasad, B.; Easterling, T.R.; Kelly, E.J.; Unadkat, J.D.; Wang, J. Effect of gestational age on mRNA and protein expression of polyspecific organic cation transporters during pregnancy. Drug Metab. Dispos. 2013, 41, 2225–2232.
- Grace, M.R.; Dotters-Katz, S.K.; Zhou, C.; Manuck, T.; Boggess, K.; Bae-Jump, V. Effect of a high-fat diet and metformin on placental mtor signaling in mice. AJP Rep. 2019, 9, e138–e143.
- Rosario, F.J.; Powell, T.; Jansson, T. Mechanistic target of rapamycin (mTOR) regulates trophoblast folate uptake by modulating the cell surface expression of FR-α and the RFC. Sci. Rep. 2016, 6.
- Jansson, T.; Aye, I.; Goberdhan, D. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta 2012, 33, e23–e29.
- Jansson, T.; Eliasson, L.; Rosario, F.; Powell, T.L.; Gupta, M.B. (Eds.) Remote control of fetal metabolism by placental mTOR signaling. In Reproductive Sciences; Sage Publications Inc.: Thousand Oaks, CA, USA, 2012; Volume 19.
- Kim, J.; Ahn, C.W.; Fang, S.; Lee, H.S.; Park, J.S. Association between metformin dose and vitamin B12 deficiency in patients with type 2 diabetes. Medicine 2019, 98, e17918.
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-term metformin use and vitamin b12 deficiency in the diabetes prevention program outcomes study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761.
- Beulens, J.W.J.; Hart, H.E.; Kuijs, R.; Kooijman-Buiting, A.M.J.; Rutten, G.E.H.M. Influence of duration and dose of metformin on cobalamin deficiency in type 2 diabetes patients using metformin. Acta Diabetol. 2015, 52, 47–53.
- Pflipsen, M.C.; Oh, R.C.; Saguil, A.; Seehusen, D.A.; Seaquist, D.; Topolski, R. The prevalence of vitamin B12 deficiency in patients with type 2 diabetes: A cross-sectional study. J. Am. Board Fam. Med. 2009, 22, 528–534.
- Reinstatler, L.; Qi, Y.P.; Williamson, R.S.; Garn, J.V.; Oakley, G.P., Jr. Association of biochemical B12 deficiency with metformin therapy and vitamin B12 supplements: The national health and nutrition examination survey, 1999–2006. Diabetes Care 2012, 35, 327–333.
- Corominas-Faja, B.; Quirantes-Piné, R.; Oliveras-Ferraros, C.; Vazquez-Martin, A.; Cufí, S.; Martin-Castillo, B.; Micol, V.; Joven, J.; Segura-Carretero, A.; Menendez, J.A. Metabolomic fingerprint reveals that metformin impairs one-carbon metabolism in a manner similar to the antifolate class of chemotherapy drugs. Aging 2012, 4, 480–498.
- Rush, E.C.; Katre, P.; Yajnik, C.S. Vitamin B12: One carbon metabolism, fetal growth and programming for chronic disease. Eur. J. Clin. Nutr. 2013, 68, 2–7.
- Maynard, A.G.; Kanarek, N. NADH ties one-carbon metabolism to cellular respiration. Cell Metab. 2020, 31, 660–662.
- Baker, B.C.; Mackie, F.L.; Lean, S.C.; Greenwood, S.L.; Heazell, A.E.P.; Forbes, K.; Jones, R.L. Placental dysfunction is associated with altered microRNA expression in pregnant women with low folate status. Mol. Nutr. Food Res. 2017, 61.
- Baker, P.N.; Wheeler, S.J.; Sanders, T.A.; Thomas, J.E.; Hutchinson, C.J.; Clarke, K.; Berry, J.L.; Jones, R.L.; Seed, P.T.; Poston, L. A prospective study of micronutrient status in adolescent pregnancy. Am. J. Clin. Nutr. 2009, 89, 1114–1124.
- Rogne, T.; Tielemans, M.J.; Chong, M.F.-F.; Yajnik, C.S.; Krishnaveni, G.V.; Poston, L.; Jaddoe, V.W.V.; Steegers, E.A.P.; Joshi, S.; Chong, Y.-S.; et al. Associations of maternal vitamin B12 concentration in pregnancy with the risks of preterm birth and low birth weight: A systematic review and meta-analysis of individual participant data. Am. J. Epidemiol. 2017, 185, 212–223.




