
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gabriel Aguirre-Álvarez | + 5677 word(s) | 5677 | 2021-05-27 07:58:22 | | | |
| 2 | Vivi Li | -2 word(s) | 5675 | 2021-06-03 04:54:08 | | |
Video Upload Options
Antioxidants are molecules that delay or inhibit the oxidation of other molecules. Its use significantly increased in recent years in the diet of people. Natural antioxidants are replacing the use of synthetic antioxidant ingredients due to their safety, nutritional, and therapeutic values. Hydrolyzed collagen (HC) is a popular ingredient considered to be an antioxidant. This low molecular weight protein has been widely utilized due to its excellent biocompatibility, easy biodegradability, and weak antigenicity. It is a safe cosmetic biomaterial with good moisturizing properties on the skin. The antioxidant properties of HC are conditioned to the size of the molecule: the lower the molecular weight of peptides, the greater the ability to donate an electron or hydrogen to stabilize radicals. The antioxidant capacity of HC is mostly due to the presence of hydrophobic amino acids in the peptide. The exact mechanism of peptides acting as antioxidants is not clearly known but some aromatic amino acids and histidine are reported to play an important role in the antioxidant activity. Oral ingestion of HC increases the levels of collagen-derived peptides in the blood torrent and improves the skin properties such as elasticity, skin moisture, and transepidermal water loss.
1. Introduction
2. Topical Formulation
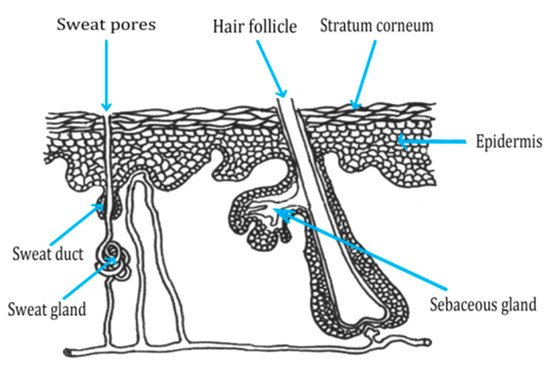
2.1. Role of the Skin in Protection
2.2. Routes of Peptide Penetration onto the Skin
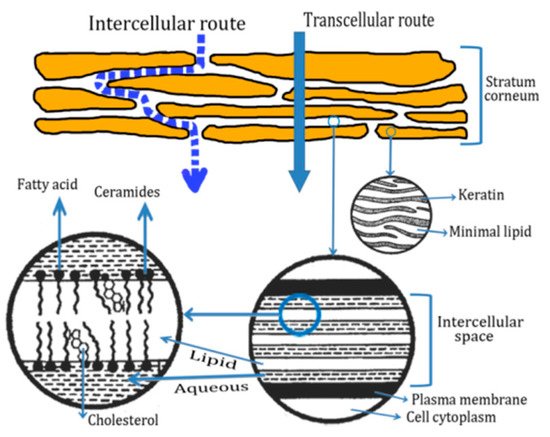
2.3. Transdermal Penetration of HC on the Skin
2.4. Anti-Aging Benefits
2.5. Creams and Lotions with Mosturizing Action
2.6. Dry Skin as a Trigger for Skin Aging
2.7. Hydration of the Skin
3. Role of Hydrolyzed Collagen as an Antioxidant Ingredient
4. Oral Administration of HC
4.1. Absorption of HC into the Blood Torrent
4.2. Improvement of Skin Properties
4.3. Protection of the Skin Against UV and Melasma
4.4. Enhancement of Fibroblast Production and Extracellular Matrix of the Skin
References
- Sies, H. Oxidative Stress: Introductory Remarks; Academic Press: London, UK, 1985; pp. 1–8.
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126.
- Fuller, B. Role of PGE-2 and Other Inflammatory Mediators in Skin Aging and Their Inhibition by Topical Natural Anti-Inflammatories. Cosmetics 2019, 6, 6.
- Levakov, A.; Vuckovic, N.; Dolai, M.; Kacanski, M.M.; Bozanic, S. Age-related skin changes. Med. Pregl. 2012, 65, 191–195.
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Neuroendocrine Aspects of Skin Aging. Int. J. Mol. Sci. 2019, 20, 2798.
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29.
- Imokawa, G. Melanocyte Activation Mechanisms and Rational Therapeutic Treatments of Solar Lentigos. Int. J. Mol. Sci. 2019, 20, 3666.
- Krutmann, J. How the sun ages our skin. The dermis as the driving force. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 2011, 62, 588–590.
- Wenk, J.; Brenneisen, P.; Meewes, C.; Wlaschek, M.; Peters, T.; Blaudschun, R.; Ma, W.; Kuhr, L.; Schneider, L.; Scharffetter-Kochanek, K. UV-induced oxidative stress and photoaging. Curr. Probl. Dermatol. 2001, 29, 83–94.
- Naidoo, K.; Birch-Machin, M.A. Oxidative Stress and Ageing: The Influence of Environmental Pollution, Sunlight and Diet on Skin. Cosmetics 2017, 4, 4.
- Rembiesa, J.; Ruzgas, T.; Engblom, J.; Holefors, A. The Impact of Pollution on Skin and Proper Efficacy Testing for Anti-Pollution Claims. Cosmetics 2018, 5, 4.
- Wlaschek, M.; Tantcheva-Poor, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schuller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B Biol. 2001, 63, 41–51.
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26.
- Eastoe, J.E.; Leach, A.A. Chemical constitution of gelatin. In The Science and Technology of Gelatin; Ward, A.G., Courts, A., Eds.; Academic Press Inc.: London, UK, 1977; pp. 73–108.
- Li, G.Y.; Fukunaga, S.; Takenouchi, K.; Nakamura, F. Comparative study of the physiological properties of collagen, gelatin and collagen hydrolysate as cosmetic materials. Int. J. Cosmet. Sci. 2005, 27, 101–106.
- Oshimura, E.; Sakamoto, K. Chapter 19—Amino Acids, Peptides, and Proteins. In Cosmetic Science and Technology; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 285–303.
- Holmes, R.; Kirk, S.; Tronci, G.; Yang, X.; Wood, D. Influence of telopeptides on the structural and physical properties of polymeric and monomeric acid-soluble type I collagen. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 823–827.
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32.
- Langmaier, F.; Mládek, M.; Kolomazník, K.; Sukop, S. Isolation of elastin and collagen polypeptides from long cattle tendons as raw material for the cosmetic industry. Int. J. Cosmet. Sci. 2002, 24, 273–279.
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557.
- Frei, V.; Perrier, E.; Orly, I.; Huc, A.; Augustin, C.; Damour, O. Activation of fibroblast metabolism in a dermal and skin equivalent model: A screening test for activity of peptides. Int. J. Cosmet. Sci. 1998, 20, 159–173.
- Schagen, S.K.; Zampeli, V.A.; Makrantonaki, E.; Zouboulis, C.C. Discovering the link between nutrition and skin aging. Derm. Endocrinol. 2012, 4, 298–307.
- Kligman, A. The future of cosmeceuticals: An interview with Albert Kligman, MD, PhD. Interview by Zoe Diana Draelos. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2005, 31, 890–891.
- Wertz, P.W. Lipids and the Permeability and Antimicrobial Barriers of the Skin. J. Lipids 2018, 2018.
- Natarajan, V.T.; Ganju, P.; Ramkumar, A.; Grover, R.; Gokhale, R.S. Multifaceted pathways protect human skin from UV radiation. Nat. Chem. Biol. 2014, 10, 542–551.
- Wertz, P.W. Lipids and barrier function of the skin. Acta Derm. Venereol. Suppl. 2000, 208, 7–11.
- Feingold, K.R. Thematic review series: Skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J. Lipid Res. 2007, 48, 2531–2546.
- Jones, L.A.; Smith, A.M. Tactile sensory system: Encoding from the periphery to the cortex. Wiley Interdiscip. Rev. Syst. Biol. Med. 2014, 6, 279–287.
- Benson, H.A. Transdermal drug delivery: Penetration enhancement techniques. Curr. Drug Deliv. 2005, 2, 23–33.
- Barry, B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2001, 14, 101–114.
- Mojumdar, E.H.; Pham, Q.D.; Topgaard, D.; Sparr, E. Skin hydration: Interplay between molecular dynamics, structure and water uptake in the stratum corneum. Sci. Rep. 2017, 7, 15712.
- Chvapli, M.; Eckmayer, Z. Role of proteins in cosmetics. Int. J. Cosmet. Sci. 1985, 7, 41–49.
- CIR. 7 Final Report on the Safety Assessment of Hydrolyzed Collagen. Cosmetic Ingredient Review. J. Am. Coll. Toxicol. 1985, 4, 199–221.
- CIR. Annual review of cosmetic ingredient safety assessments-2004/2005. Int. J. Toxicol. 2006, 25, 1–89.
- CIR. Safety Assessment of Skin and Connective Tissue-Derived Proteins and Peptides as Used in Cosmetics; Cosmetic Ingredient Review: Washington, DC, USA, 2017; pp. 1–28.
- Lintner, K. Peptides and proteins. In Cosmetic Dermatology, Products and Procedures; Draelos, Z.D., Ed.; Wiley-Blackwell: West Sussex, UK, 2010; pp. 292–301.
- Ranade, V.V. Drug delivery systems. 6. Transdermal drug delivery. J. Clin. Pharmacol. 1991, 31, 401–418.
- Gorouhi, F.; Maibach, H.I. Role of topical peptides in preventing or treating aged skin. Int. J. Cosmet. Sci. 2009, 31, 327–345.
- Zhang, H.; Pan, D.; Dong, Y.; Su, W.; Su, H.; Wei, X.; Yang, C.; Jing, L.; Tang, X.; Li, X.; et al. Transdermal permeation effect of collagen hydrolysates of deer sinew on mouse skin, ex vitro, and antioxidant activity, increased type I collagen secretion of percutaneous proteins in NIH/3T3 cells. J. Cosmet. Dermatol. 2019.
- Secchi, G. Role of protein in cosmetics. Clin. Dermatol. 2008, 26, 321–325.
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2017, 28, 4–9.
- Chai, H.J.; Li, J.H.; Huang, H.N.; Li, T.L.; Chan, Y.L.; Shiau, C.Y.; Wu, C.J. Effects of sizes and conformations of fish-scale collagen peptides on facial skin qualities and transdermal penetration efficiency. J. Biomed. Biotechnol. 2010, 2010, 757301.
- Byrne, A.J.; Al-Bader, T.; Kerrigan, D.; Hickey, S.; Laloeuf, A.; Rawlings, A.V. Synergistic action of a triple peptide complex on an essential extra-cellular matrix protein exhibits significant anti-aging benefits. J. Cosmet. Dermatol. 2010, 9, 108–116.
- Trookman, N.S.; Rizer, R.L.; Ford, R.; Ho, E.; Gotz, V. Immediate and Long-term Clinical Benefits of a Topical Treatment for Facial Lines and Wrinkles. J. Clin. Aesthet. Derm. 2009, 2, 38–43.
- Swatschek, D.; Schatton, W.; Kellermann, J.; Muller, W.E.; Kreuter, J. Marine sponge collagen: Isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. 2002, 53, 107–113.
- Berardesca, E.; Abril, E.; Serio, M.; Cameli, N. Effects of topical gluco-oligosaccharide and collagen tripeptide F in the treatment of sensitive atopic skin. Int. J. Cosmet. Sci. 2009, 31, 271–277.
- Lupo, M.P.; Cole, A.L. Cosmeceutical peptides. Dermatol. Ther. 2007, 20, 343–349.
- Bode, W.; Fernandez-Catalan, C.; Grams, F.; Gomis-Ruth, F.X.; Nagase, H.; Tschesche, H.; Maskos, K. Insights into MMP-TIMP interactions. Ann. N. Y. Acad. Sci. 1999, 878, 73–91.
- Xhauflaire-Uhoda, E.; Fontaine, K.; Piérard, G.E. Kinetics of moisturizing and firming effects of cosmetic formulations. Int. J. Cosmet. Sci. 2008, 30, 131–138.
- Sethi, A.; Kaur, T.; Malhotra, S.; Gambhir, M. Moisturizers: The slippery road. Indian J. Dermatol. 2016, 61, 279–287.
- Flynn, T.C.; Petros, J.; Clark, R.E.; Viehman, G.E. Dry skin and moisturizers. Clin. Dermatol. 2001, 19, 387–392.
- Kraft, J.N.; Lynde, C.W. Moisturizers: What they are and a practical approach to product selection. Ski. Ther. Lett. 2005, 10, 1–8.
- Spada, F.; Barnes, T.M.; Greive, K.A. Skin hydration is significantly increased by a cream formulated to mimic the skin’s own natural moisturizing systems. Clin. Cosmet. Investig. Dermatol. 2018, 11, 491–497.
- Ciszek, A. Variability of skin pH after the use of different collagen gels. J. Cosmet. Dermatol. 2017, 16, 531–536.
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294.
- Zanna, N. Defeating dry skin: Treatments, topical ingredients and the role of nutrition. J. Aesthet. Nurs. 2015, 4, 478–485.
- Duplan, H.; Nocera, T. Skin hydration and hydrating products. Ann. Dermatol. Venereol. 2018, 145, 376–384.
- Gioia, F.; Celleno, L. The dynamics of transepidermal water loss (TEWL) from hydrated skin. Ski. Res. Technol. 2002, 8, 178–186.
- Bernengo, J.-C.; de Rigal, J. Physical Methods to Measure Stratum Corneum Water Content In Vivo. In Agache’s Measuring the Skin: Non-Invasive Investigations, Physiology, Normal Constants; Humbert, P., Fanian, F., Maibach, H.I., Agache, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 299–340.
- Milani, M.; Sparavigna, A. The 24-hour skin hydration and barrier function effects of a hyaluronic 1%, glycerin 5%, and Centella asiatica stem cells extract moisturizing fluid: An intra-subject, randomized, assessor-blinded study. Clin. Cosmet. Investig. Dermatol. 2017, 10, 311–315.
- Voegeli, R.; Gierschendorf, J.; Summers, B.; Rawlings, A.V. Facial skin mapping: From single point bio-instrumental evaluation to continuous visualization of skin hydration, barrier function, skin surface pH, and sebum in different ethnic skin types. Int. J. Cosmet. Sci. 2019, 41, 411–424.
- Guzmán-Alonso, M.; Cortazár, T.M. Water content at different skin depths and the influence of moisturizing formulations. Househ. Pers. Care Today 2016, 11, 35–40.
- Bielfeldt, S.; Schoder, V.; Ely, U.; Van Der Pol, A.; De Sterke, J.; Wilhelm, K.-P. Assessment of human stratum corneum thickness and its barrier properties by in-vivo confocal Raman spectroscopy. Int. J. Cosmet. Sci. 2009, 31, 479–480.
- Verdier-Sevrain, S.; Bonte, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82.
- Gniadecka, M.; Faurskov Nielsen, O.; Christensen, D.H.; Wulf, H.C. Structure of water, proteins, and lipids in intact human skin, hair, and nail. J. Investig. Dermatol. 1998, 110, 393–398.
- Waller, J.M.; Maibach, H.I. Age and skin structure and function, a quantitative approach (II): Protein, glycosaminoglycan, water, and lipid content and structure. Ski. Res. Technol. 2006, 12, 145–154.
- Qassem, M.; Kyriacou, P. Review of Modern Techniques for the Assessment of Skin Hydration. Cosmetics 2019, 6, 19.
- Egawa, M. In vivo simultaneous measurement of urea and water in the human stratum corneum by diffuse-reflectance near-infrared spectroscopy. Ski. Res. Technol. 2009, 15, 195–199.
- Wang, H.; Zhang, Q.; Mao, G.; Conroy, O.; Pyatski, Y.; Fevola, M.J.; Cula, G.O.; Maitra, P.; Mendelsohn, R.; Flach, C.R. Novel confocal Raman microscopy method to investigate hydration mechanisms in human skin. Skin. Res. Technol. 2019, 25, 653–661.
- Huang, X.; Yeo, W.H.; Liu, Y.; Rogers, J.A. Epidermal differential impedance sensor for conformal skin hydration monitoring. Biointerphases 2012, 7, 52.
- Webb, R.C.; Bonifas, A.P.; Behnaz, A.; Zhang, Y.; Yu, K.J.; Cheng, H.; Shi, M.; Bian, Z.; Liu, Z.; Kim, Y.-S.; et al. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 2013, 12, 938–944.
- Krishnan, S.; Shi, Y.; Webb, R.C.; Ma, Y.; Bastien, P.; Crawford, K.E.; Wang, A.; Feng, X.; Manco, M.; Kurniawan, J.; et al. Multimodal epidermal devices for hydration monitoring. Microsyst. Amp Nanoeng. 2017, 3, 17014.
- Woo, Y.-A.; Ahn, J.-W.; Chun, I.-K.; Kim, H.-J. Development of a Method for the Determination of Human Skin Moisture Using a Portable Near-Infrared System. Anal. Chem. 2001, 73, 4964–4971.
- Fluhr, J.W.; Darlenski, R. Transepidermal Water Loss (TEWL). In Non Invasive Diagnostic Techniques in Clinical Dermatology; Berardesca, E., Maibach, H.I., Wilhelm, K.-P., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 353–356.
- Lopez-Alarcon, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10.
- Hermund, D.B. Antioxidant properties of seaweed-derived substances. In Bioactive Seaweeds for Food Applications, Natural Ingredients for Healty Diets; Qin, Y., Ed.; Academic Press: London, UK, 2018; pp. 201–222.
- Silva, S.; Ferreira, M.; Oliveira, A.S.; Magalhães, C.; Sousa, M.E.; Pinto, M.; Sousa Lobo, J.M.; Almeida, I.F. Evolution of the use of antioxidants in anti-ageing cosmetics. Int. J. Cosmet. Sci. 2019, 41, 378–386.
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon 2019, 5, e01538.
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9.
- Hettiarachchy, N.S.; Glenn, K.C.; Gnanasambandam, R.; Johnson, M.G. Natural Antioxidant Extract from Fenugreek (Trigonella foenumgraecum) for Ground Beef Patties. J. Food Sci. 1996, 61, 516–519.
- León-López, A.; Fuentes-Jiménez, L.; Hernández-Fuentes, A.D.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Hydrolysed Collagen from Sheepskins as a Source of Functional Peptides with Antioxidant Activity. Int. J. Mol. Sci. 2019, 20, 3931.
- Mendis, E.; Rajapakse, N.; Byun, H.G.; Kim, S.K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 77, 2166–2178.
- Qian, Z.J.; Jung, W.K.; Byun, H.G.; Kim, S.K. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour. Technol. 2008, 99, 3365–3371.
- Rajapakse, N.; Mendis, E.; Jung, W.K.; Je, J.Y.; Kim, S.K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182.
- Kim, S.Y.; Je, J.Y.; Kim, S.K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38.
- Je, J.Y.; Qian, Z.J.; Lee, S.H.; Byun, H.G.; Kim, S.K. Purification and antioxidant properties of bigeye tuna (Thunnus obesus) dark muscle peptide on free radical-mediated oxidative systems. J. Med. Food 2008, 11, 629–637.
- Slizyte, R.; Mozuraityte, R.; Martinez-Alvarez, O.; Falch, E.; Fouchereau-Peron, M.; Rustad, T. Functional, bioactive and antioxidative properties of hydrolysates obtained from cod (Gadus morhua) backbones. Process Biochem. 2009, 44, 668–677.
- Thinsilakul, Y.; Benjakul, S.; Shahidi, F. Antioxidative activity of protein hydrolysate from round scad muscle using alcalase and flavourzyme. J. Food Biochem. 2007, 31, 266–287.
- Amarowicz, R.; Shahidi, F. Antioxidant activity of peptide fractions of capelin protein hydrolysates. Food Chem. 1997, 58, 355–359.
- Wu, H.C.; Chen, H.M.; Shiau, C.Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957.
- Cho, S.-S.; Hyo-ku, L.; Yeon, Y.C.; Kim, M.J.; Soo, S.E.; Eun-Hwa, S.; Myoung-Gun, C.; Lim, J.D. Isolation and characterization of bioactive peptides from Hwangtae (yellowish dried Alaska pollack) protein hydrolysate. J. Food Sci. Nutr. 2008, 13, 196–203.
- Jun, S.-Y.; Park, P.-J.; Jung, W.-K.; Kim, S.-K. Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limanda aspera) frame protein. Eur. Food Res. Technol. 2004, 219, 20–26.
- Klompong, V.; Benjakul, S.; Yachai, M.; Visessanguan, W.; Shahidi, F.; Hayes, K.D. Amino acid composition and antioxidative peptides from protein hydrolysates of yellow stripe Trevally (Selaroides leptolepis). J. Food Sci. 2009, 74, C126–C133.
- Kim, S.-K.; Wijesekara, I.; Park, E.Y.; Matsumura, Y.; Nakamura, Y.; Sato, K. Proteins and peptides as antioxidants. In Bioactive Food Proteins and Peptides, Applications in Human Health; Hettiarachchy, N.S., Sato, K., Marshall, M.R., Kannan, A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 97–116.
- Marini, A. Beauty from the inside. Does it really work? Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 2011, 62, 614–617.
- Draelos, Z.D. Aging skin: The role of diet: Facts and controversies. Clin. Dermatol. 2013, 31, 701–706.
- Taeymans, J.; Clarys, P.; Barel, A.O. Use of Food Supplements as Nutricosmetics in Health and Fitness: A Review. In Handbook of Cosmetic Science and Technology, 4th ed.; Barel, A.O., Paye, M., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 583–596.
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536.
- Ichikawa, S.; Morifuji, M.; Ohara, H.; Matsumoto, H.; Takeuchi, Y.; Sato, K. Hydroxyproline-containing dipeptides and tripeptides quantified at high concentration in human blood after oral administration of gelatin hydrolysate. Int. J. Food Sci. Nutr. 2010, 61, 52–60.
- Ohara, H.; Matsumoto, H.; Ito, K.; Iwai, K.; Sato, K. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J. Agric. Food Chem. 2007, 55, 1532–1535.
- Yazaki, M.; Ito, Y.; Yamada, M.; Goulas, S.; Teramoto, S.; Nakaya, M.A.; Ohno, S.; Yamaguchi, K. Oral Ingestion of Collagen Hydrolysate Leads to the Transportation of Highly Concentrated Gly-Pro-Hyp and Its Hydrolyzed Form of Pro-Hyp into the Bloodstream and Skin. J. Agric. Food Chem. 2017, 65, 2315–2322.
- Skov, K.; Oxfeldt, M.; Thogersen, R.; Hansen, M.; Bertram, H.C. Enzymatic Hydrolysis of a Collagen Hydrolysate Enhances Postprandial Absorption Rate-A Randomized Controlled Trial. Nutrients 2019, 11, 1064.
- López-Morales, C.A.; Vázquez-Leyva, S.; Vallejo-Castillo, L.; Carballo-Uicab, G.; Muñoz-García, L.; Herbert-Pucheta, J.E.; Zepeda-Vallejo, L.G.; Velasco-Velázquez, M.; Pavón, L.; Pérez-Tapia, S.M.; et al. Determination of Peptide Profile Consistency and Safety of Collagen Hydrolysates as Quality Attributes. J. Food Sci. 2019, 84, 430–439.
- Osawa, Y.; Mizushige, T.; Jinno, S.; Sugihara, F.; Inoue, N.; Tanaka, H.; Kabuyama, Y. Absorption and metabolism of orally administered collagen hydrolysates evaluated by the vascularly perfused rat intestine and liver in situ. Biomed. Res. (Tokyo Jpn.) 2018, 39, 1–11.
- Yamamoto, S.; Hayasaka, F.; Deguchi, K.; Okudera, T.; Furusawa, T.; Sakai, Y. Absorption and plasma kinetics of collagen tripeptide after peroral or intraperitoneal administration in rats. Biosci. Biotechnol. Biochem. 2015, 79, 2026–2033.
- Genovese, L.; Corbo, A.; Sibilla, S. An Insight into the Changes in Skin Texture and Properties following Dietary Intervention with a Nutricosmeceutical Containing a Blend of Collagen Bioactive Peptides and Antioxidants. Ski. Pharmacol. Physiol. 2017, 30, 146–158.
- Inoue, N.; Sugihara, F.; Wang, X. Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial ageing signs in a randomised double-blind placebo-controlled clinical study. J. Sci. Food Agric. 2016, 96, 4077–4081.
- Sugihara, F.; Inoue, N. Clinical effects of collagen hydrolysates ingestion on UV-induced pigmented spots of human skin: A preliminary study. Health Sci. 2012, 28, 153–156.
- Ito, N.; Seki, S.; Ueda, F. Effects of Composite Supplement Containing Collagen Peptide and Ornithine on Skin Conditions and Plasma IGF-1 Levels-A Randomized, Double-Blind, Placebo-Controlled Trial. Mar Drugs 2018, 16, 482.
- Matsumoto, H. Clinical effects of fish type I collagen hydrolysate on skin properties. ITE Lett. Batter. New Technol. Med. 2006, 7, 386–390.
- Schwartz, S.R.; Park, J. Ingestion of BioCell Collagen®, a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs. Clin. Interv. Aging 2012, 7, 267–273.
- Petersen Vitello Kalil, C.L.; Campos, V.; Cignachi, S.; Favaro Izidoro, J.; Prieto Herman Reinehr, C.; Chaves, C. Evaluation of cutaneous rejuvenation associated with the use of ortho-silicic acid stabilized by hydrolyzed marine collagen. J. Cosmet. Dermatol. 2018, 17, 814–820.
- Tanaka, M.; Koyama, Y.; Nomura, Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci. Biotechnol. Biochem. 2009, 73, 930–932.
- Gui, M.; Kan, J.; Qu, D.; Chen, Y.; Luo, R.; Liu, Y.; Du, J. Instrumental Evaluation of the Depigmenting Efficacy of an Oral Supplementation Containing Peptides and Chrysanthemum Extract for the Treatment of Melasma. Cosmetics 2017, 4, 42.
- Lee, H.-J.; Jang, H.-L.; Ahn, D.-K.; Kim, H.-J.; Jeon, H.Y.; Seo, D.B.; Lee, J.-H.; Choi, J.K.; Kang, S.-S. Orally administered collagen peptide protects against UVB-induced skin aging through the absorption of dipeptide forms, Gly-Pro and Pro-Hyp. Biosci. Biotechnol. Biochem. 2019, 83, 1146–1156.
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking older: Fibroblast collapse and therapeutic implications. Arch. Dermatol. 2008, 144, 666–672.
- Edgar, S.; Hopley, B.; Genovese, L.; Sibilla, S.; Laight, D.; Shute, J. Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Sci. Rep. 2018, 8, 10474.
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of Dermis: Scarring and Cells Involved. Cells 2019, 8, 607.
- Asai, T.T.; Oikawa, F.; Yoshikawa, K.; Inoue, N.; Sato, K. Food-Derived Collagen Peptides, Prolyl-Hydroxyproline (Pro-Hyp), and Hydroxyprolyl-Glycine (Hyp-Gly) Enhance Growth of Primary Cultured Mouse Skin Fibroblast Using Fetal Bovine Serum Free from Hydroxyprolyl Peptide. Int. J. Mol. Sci. 2019, 21, 229.
- Marcos-Garcés, V.; Molina Aguilar, P.; Bea Serrano, C.; García Bustos, V.; Benavent Seguí, J.; Ferrández Izquierdo, A.; Ruiz-Saurí, A. Age-related dermal collagen changes during development, maturation and ageing—A morphometric and comparative study. J. Anat. 2014, 225, 98–108.
- Proksch, E.; Schunck, M.; Zague, V.; Segger, D.; Degwert, J.; Oesser, S. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Ski. Pharmacol. Physiol. 2014, 27, 113–119.
- Kielty, C.M.; Sherratt, M.J.; Shuttleworth, C.A. Elastic fibres. J. Cell Sci. 2002, 115, 2817–2828.
- Zague, V.; de Freitas, V.; da Costa Rosa, M.; de Castro, G.A.; Jaeger, R.G.; Machado-Santelli, G.M. Collagen hydrolysate intake increases skin collagen expression and suppresses matrix metalloproteinase 2 activity. J. Med. Food 2011, 14, 618–624.
- Liang, J.; Pei, X.; Zhang, Z.; Wang, N.; Wang, J.; Li, Y. The protective effects of long-term oral administration of marine collagen hydrolysate from chum salmon on collagen matrix homeostasis in the chronological aged skin of Sprague-Dawley male rats. J. Food Sci. 2010, 75, H230–H238.
- Liu, Z.; Li, Y.; Song, H.; He, J.; Li, G.; Zheng, Y.; Li, B. Collagen peptides promote photoaging skin cell repair by activating the TGF-beta/Smad pathway and depressing collagen degradation. Food Funct. 2019, 10, 6121–6134.
- Borumand, M.; Sibilla, S. Daily consumption of the collagen supplement Pure Gold Collagen® reduces visible signs of aging. Clin. Interv. Aging 2014, 9, 1747–1758.
- Asserin, J.; Lati, E.; Shioya, T.; Prawitt, J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015, 14, 291–301.




