Antioxidants are molecules that delay or inhibit the oxidation of other molecules. Its use significantly increased in recent years in the diet of people. Natural antioxidants are replacing the use of synthetic antioxidant ingredients due to their safety, nutritional, and therapeutic values. Hydrolyzed collagen (HC) is a popular ingredient considered to be an antioxidant. This low molecular weight protein has been widely utilized due to its excellent biocompatibility, easy biodegradability, and weak antigenicity. It is a safe cosmetic biomaterial with good moisturizing properties on the skin. The antioxidant properties of HC are conditioned to the size of the molecule: the lower the molecular weight of peptides, the greater the ability to donate an electron or hydrogen to stabilize radicals. The antioxidant capacity of HC is mostly due to the presence of hydrophobic amino acids in the peptide. The exact mechanism of peptides acting as antioxidants is not clearly known but some aromatic amino acids and histidine are reported to play an important role in the antioxidant activity. Oral ingestion of HC increases the levels of collagen-derived peptides in the blood torrent and improves the skin properties such as elasticity, skin moisture, and transepidermal water loss.
- peptide
- skincare
- extracellular matrix
- hydrolyzed collagen
- skin aging
1. Introduction
2. Topical Formulation
2.1. Role of the Skin in Protection
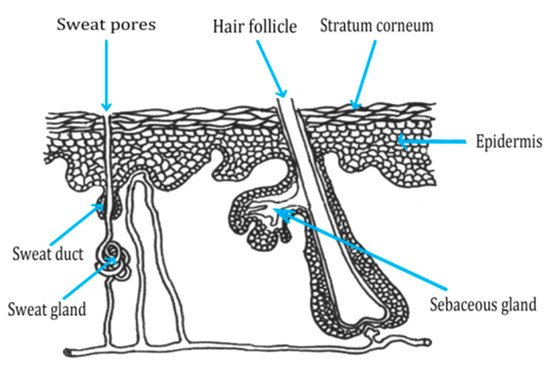
2.2. Routes of Peptide Penetration onto the Skin
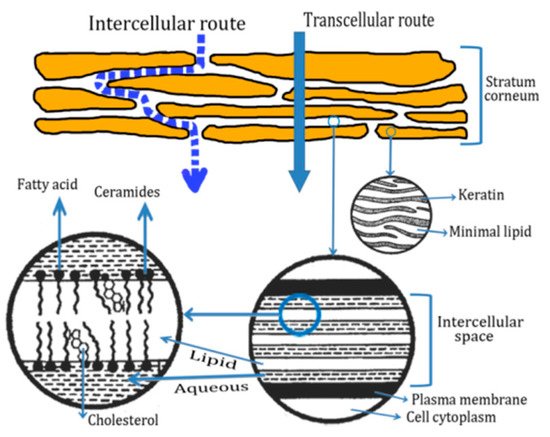
2.3. Transdermal Penetration of HC on the Skin
2.4. Anti-Aging Benefits
2.5. Creams and Lotions with Mosturizing Action
2.6. Dry Skin as a Trigger for Skin Aging
2.7. Hydration of the Skin
3. Role of Hydrolyzed Collagen as an Antioxidant Ingredient
4. Oral Administration of HC
4.1. Absorption of HC into the Blood Torrent
Beauty comes from the inside and that means that nutrition is a key point for healthy skin and therefore decelerating the skin aging process [95,96]. Ingestible food products that are formulated for beauty purposes are described now as "nutricosmetics" [97].
Several works have demonstrated that the levels of collagen-derived peptides in the blood torrent increased significantly after HC oral ingestion, suggesting that collagen molecules are absorbed into human plasma [98,99]. Ohara et al. [100] formulated a study in five healthy males looking at a comparison in quantity and structure of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from fish and skin sources. They concluded that 30% of all detected hydroxyproline (Hyp) corresponded to Hyp-containing peptides compared to the free form of Hyp. Yazaky and co-workers [101] also analyzed the plasma concentrations of collagen-derived peptides into the bloodstream and skin in twelve healthy individuals who ingested (300 mg/Kg body weight) either high tri-peptide containing collagen hydrolysates (HTC-Col) or ow tri-peptide containing collagen hydrolysates (LTC-Col). They concluded that HTC-Co showed peak richness in tri-peptide components in the blood after oral ingestion (13.6%).
In 2019, Skov et al. [102] carried out a clinical study to investigate the postprandial (after eating a meal) absorption of collagen from beef bone and elucidated the impact of exogenous enzymatic hydrolysis on absorption rate and bioavailability. Ten healthy male subjects ingested either 35 g enzymatically hydrolyzed collagen protein (EHC), non-enzymatically hydrolyzed collagen (NC), or placebo (250 mL water) during three consecutive days. The study concluded that the absorption rate and bioavailability of three particular amino acids (Gly, Pro, and Hyp) were significantly higher after oral administration of EHC. The results gathered suggested that ingestion of collagen in its hydrolyzed form promotes a higher absorption rate in the postprandial plasma concentration of total amino acids compared to the NC and placebo group.
Human consumption of dietary supplements such as hydrolyzed collagen has been deemed to be safe. Lopez-Morales et al. [103] conducted a detailed experimental work to characterize HC with different techniques such as ultraviolet detection (SEC–UV), reverse phase chromatography coupled to electrospray ionization ion mobility quadrupole time-of-flight spectrometer (RP–HPLCESI–IMS–QTOF), and shaped-pulse water-suppression one (1D)- and two-dimensional (2D) proton nuclear magnetic resonance spectra. Safety and toxicity of HC were assessed in vitro by using CaCo-2 and HepG2 cell lines. They found that HC is safe and no toxic on the evaluated cell lines. The mass distribution pattern obtained by SEC resulted in a range from 1.35 kDa to 17 kDa and RP–HPLCESI–IMS–QTOF showed a range from 2 kDa to 14 kDa. These low molecular weight peptides were soluble in water and able to be digested, absorbed, and transported to the systemic circulation system as peptides in the small intestine [104]. The absorption of HC tripeptides (Gly-Pro-Hyp) or dipeptides (Pro-Hyp) in the bloodstream was demonstrated in rats’ model by Yamamoto and co-workers. This occurs as early as 10 minutes after oral administration [105].
4.2. Improvement of Skin Properties
In 2017, Genovese et al. [106] performed a clinical study of 120 healthy volunteers for 90 days. They were instructed to ingest either 50 mL of nutricosmetic formulation (hydrolyzed collagen, hyaluronic acid and N-acetylglucosamine, borage oil, and other ingredients such as vitamins, minerals antioxidants, and additional bioactive ingredients) or 50 mL of placebo (water and other ingredients such as flavors, organic acids, and soybean polysaccharide). The histological analysis revealed that oral supplementation of the nutricosmetic formulation produced an improvement in the structure and stratification of the epidermal layers. The collagen fibers’ structural architecture within the dermis was improved. A complementary part of this study was the self-assessment questionnaire. The most relevant answers commented that 95% of the subjects agreed their skin was more hydrated. They answered their skin was more elastic (91.6%), stronger (81.7%), and thicker (91.7%).
Another clinical study was conducted in 85 Chinese female subjects treated to low and high free-formed ratios of Pro-Hyp and Hyp-Gly derived from fish source [107]. Five-gram samples were ingested for eight weeks. Three physiological measurements were evaluated on the skin; the skin moisture showed a significant increase compared to the placebo. In regard to skin elasticity, a significant improvement of facial skin elasticity after oral ingestion of collagen hydrolysates resulted. The higher ratios of Pro-Hyp and Hyp-Gly showed the best results in skin elasticity. This treatment also appeared to have the best results for surface skin measurements because of a significant reduction in the number of wrinkles, wrinkle area, wrinkle depth, and roughness. Other works from these authors also discovered that oral ingestion of HC decreased the area of UV spots on the skin after four weeks of oral ingestion [108].
Similar results on the improvement of the skin were obtained by Ito et al. [109] in 40 healthy women by the ingestion of 30-mL of fish-derived collagen and ornithine (CPO) drink. After eight weeks of supplementation, skin elasticity increased in CPO treatments. Skin moisture and TEWL were significantly attenuated on CPO groups compared with placebo. In addition, the number of skin pores was reduced in CPO treatment.
Improvements on skin properties were reported previously [110] in a clinical study carried out with fish HC administered to 25 Japanese female subjects diagnosed with dry and rough skin. The results showed that the moisture content of the SC of face-cheek, forearm, and the back of the neck increased significantly after six weeks of daily intake of 7 g of HC. Also, there were significant enhancements in skin elasticity by reducing wrinkles and lowering skin roughness.
Skin aging produces physiological changes at the dermis level and is manifested by visible signs such as dryness, laxity, and wrinkles and photoaging in the face. Schwartz and Park [111] developed a study to treat these effects of skin aging. A 1 g mixture of hydrolyzed collagen type II from chicken sternal articular cartilage, hyaluronic acid, and chondroitin sulfate was administered to 26 healthy females for twelve weeks. The study showed that oral supplementation led to a significant decrease in facial lines and wrinkles including skin dryness and scaling.
The benefits of ortho-silicic acid stabilized in hydrolyzed marine collagen as a treatment against the skin aging process were evaluated in another study [112]. Twenty-two female and male volunteers were counseled to take, before breakfast, one capsule daily with ortho-silicic acid and hydrolyzed marine collagen. After 90 days of treatment, the authors concluded that degenerative changes of the ECM were recovered, stimulation for the synthesis of collagen type I, improvements in skin firmness, texture, and hydration were achieved.
4.3. Protection of the Skin Against UV and Melasma
Ultraviolet is divided into three regions according to wavelength: UV-A (400–315 nm), UV-B (315–280 nm), and UV-C (<280). Continuous exposition to UV-B results in an aged skin with wrinkle formation [113]. The benefits of a novel oral supplement (CP) prepared with fish skin HC combined with soy peptides and aqueous extract of Flos Chrysanthemi Alba were investigated in a clinical study to evaluate the safety and efficacy of CP to treat melasma [114]. Sixty-two healthy female volunteers who were diagnosed with melasma by a dermatologist were included in the study. They were instructed to take 10 g of CP per day along with breakfast for a period of 60 days. The authors reported a reduction in hyperpigmentation with lighter facial skin tone after oral supplementation of CP. The melanin contrast index in the lesion area was reduced in comparison with the placebo group. Their results suggested that CP inhibited UV-B-induced pigmentation because of the antioxidant activity and tyrosinase inhibitory effects of CP. Lee et al. conducted research to study the effects of oral administration of fish scale collagen peptides on the skin protection when exposed to UV-B. They concluded that consumption of dipeptides in the form of Gly-Pro and Pro-Hyp attenuated the UV-B induced wrinkle formation. The enhancement of other skin properties was reported such as skin hydration, transepidermal water loss, and epidermis thickness [115].
4.4. Enhancement of Fibroblast Production and Extracellular Matrix of the Skin
The extracellular matrix (ECM) is made up of several types of proteins such as collagen type I, elastin, and proteoglycans, which are largely produced and secreted for fibroblasts [116,117]. Some of the changes from the aging of the skin are the structural modifications of the reticular dermis induced by loss of internal cohesion as well as the rupture and decrease of collagen and elastin fibers. This happens because there is reduced production of fibroblasts responsible for the production of collagen, and collagen fibers are fragmented and thinned [118]. However, daily intakes of food-derived collagen peptides enhance the growth of fibroblasts. Pro-Hyp and Hyp-Gly dipeptides play an important role in the proliferation of fibroblasts [119]. It has been demonstrated that the rest of the fragmented collagen inhibits synthesis of procollagen by fibroblasts, blocks their proliferation, induces a senescent fibroblastic morphology, and stimulates the expression of matrix metalloproteinases (MMP) [120]. These defects impair the structural and mechanical integrity of the dermis and, therefore, alter its functions; reduction of glycosaminoglycans content and consequent loss of water retention in the dermis due to the reduction of hygroscopic capacity of the skin. All these morphological and physiological changes are manifested in the skin as flaccidity and loss of texture.
In 2014, Proksch et al. [121] carried out a clinical study in 114 healthy female subjects to investigate the effect of the oral ingestion of 2.5 g porcine type I collagen peptides with an average Mw of 2 kDa for a period of time of eight weeks. The eye wrinkle volume parameter showed good results after four weeks of treatment. However, after eight weeks, the reduction of wrinkles reached up to 20% on average compared to the placebo. Production of procollagen type I and elastin were increased by up to 65% and 18%, respectively, after eight weeks of treatment. Fibril content increased by up to 6% compared to the placebo. It is well-known that elastic fibers are essential ECM molecules composing an elastin core surrounding by a mantle of fibrillin-rich microfibrils [122]. Previous studies have demonstrated that daily intake of collagen peptides decreases the expression levels of matrix metalloproteinase, which is responsible for collagen breakdown [123, 124,125].
Borumand and Sibilla conducted research oriented to investigating the effect of a supplement composed of collagen hydrolysates from tilapia and pangasius fish, hyaluronic acid, vitamins, and minerals on skin properties [126]. The study was carried out in 294 volunteers aged 18–74 years old recruited by 40 dermatologists across five different countries. They were instructed to drink 50 mL of their food supplement once a day. The study was structured into three sections. The first section reported that 69% had either visible or significant improvement in their facial lines after 60 days of treatment. The volunteers improved their facial photoaging problems and skin dryness. The second section reported an increment in dermal collagen density. After 12 weeks of treatment, the magnitude of collagen density was greater on the face than on the forearm. The third section showed that the skin firmness increased by up to 94% when the treatment reached 130 days.
Aserrin and co-workers completed a study to assess the effect of daily oral supplementation with collagen peptides on skin hydration, collagen density, and collagen fragmentation. Human skin explants were used to study the ECM components [127]. Thirty-three female volunteers, 40–59 years old, were recruited to ingest 10 g of either fish collagen, porcine collagen, or placebo. Porcine collagen showed the best hydration increasing by up to 28% after eight consecutive weeks of intake. Results from the high-frequency ultrasound demonstrated that oral intake of collagen peptides increased the dermal echogenicity significantly immediately after four weeks of treatment. Additionally, this effect persisted after twelve weeks of treatment when compared with the baseline value. Collagen peptides significantly reduced fragmentation after supplementation. Fibers in the skin appear to be larger collagen fragments compared to the placebo and baseline values. The general morphology, glycosaminoglycans, and collagen content of the human skin explants reported that the glycosaminoglycan level in the basal epidermis increased significantly. In correlation with these results, the collagen content of the papillary dermis increased in response to the incubation with collagen peptides. However, the general morphology of the skin was not affected significantly.
This entry is adapted from the peer-reviewed paper 10.3390/antiox9020181
