
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Omar Mertins | + 1310 word(s) | 1310 | 2020-05-29 13:20:54 | | | |
| 2 | Catherine Yang | Meta information modification | 1310 | 2020-06-10 11:06:00 | | | | |
| 3 | Catherine Yang | -17 word(s) | 1293 | 2020-10-27 11:54:57 | | |
Video Upload Options
Cubosomes are soft biocontinuous nanoparticles whose 3D geometry can be engineered to render the structures responsive to pH variations, which is of large interest to the production of efficient drug delivery materials. We have reviewed the literature to provide a state of the art in this regard and shed lights on prominent perspectives and strategies for pH-sensitive cubosomes development, taking advantage of the pH changes of the biological media at targeted application sites.
1. Introduction
Lipid nanoparticle-mediated drug delivery experiences rapid developments in the field of liquid crystalline colloidal carriers, e.g., cubosomes, spongosomes, hexosomes and vesicles [1][2][3][4][5][6][7]. In addition to the expansion of liposomes as advanced drug delivery systems [8][9][10], a plethora of research has been dedicated to diverse mesoporous liquid crystalline materials and nanostructures intended as drug delivery devices [11][12][13][14][15][16]. Lipid-based cubic mesophases are increasingly implemented in noninvasive drug delivery applications [17][18][19]. The liquid crystalline structure of cubosomes, consisting of well-defined networks of aqueous channels and lipid bilayer membranes, organized in periodic 3D topologies, presents advantages over other delivery systems [20]. Scientific and technological advances have been achieved in the fabrication of biocompatible systems for encapsulation of natural and synthetic lipophilic, hydrophilic and hydrophobic drug molecules, a variety of macromolecular drugs (peptides, proteins, DNA, siRNA, etc.) and imaging agents [1][2][3][4][5][6][7][11][12][13][14][15][16][17][18][19][21][22][23][24]. Complex cubic lattice networks of high surface areas provide enhanced protection of the incorporated payload from degradation as well as the prolonged and sustained release of the entrapped bioactive molecules [25][26].
Current demands for improved performance and specificity of drug delivery carriers require the use of intelligent materials, which respond to various environmental stimuli [27]. In this context, the cubosome assemblies present further advantages because the transformations between the different liquid crystalline organizations, e.g., Pn3m, Im3m and Ia3d, besides the inverted hexagonal phases (Figure 1), can be tuned and controlled by changes in temperature, ionic strength or pH of the environment of the targeted application sites [28][29][30][31][32][33][34].
2. Recent advances
In the present work, we review recent advances in cubosome nanocarriers and bulk cubic mesophases with a particular emphasis on the pH effects on the structures and topologies of the designed drug delivery systems. This interest is motivated by the fact that pH represents an inherent physiological condition of all biological organisms and that pathological (inflamed or infected) areas can embed target sites for pH-sensitive nanomedicines. This has led to the emergence of pH-responsive drug delivery carriers and nanomaterials, some of which are reviewed here below.
Acidic compartments of the cells are the endosomes (pH 5–6) and the lysosomes (pH 5). Depending on the application site, pH changes of the biological medium may comprise a profitable condition to boost the target release of encapsulated drugs from the pH-responsive nanocarriers. In this context, lipid-based pH-sensitive cubosomes have been produced by the assembly of traditional cubic-phase-forming amphiphiles monoolein or phytantriol with charged or ionizable lipids [35][36][37][38][39][40][41][42]. Incorporation of drugs like doxorubicin, for which the redox process (Figure 1) provokes pH-dependent structural changes, may also lead to pH-responsiveness of the host cubic phase carriers. Furthermore, the association of lipids with polyelectrolytes and charged surfactants was employed as a means to generate highly pH-sensitive cubosomes and other types of nanocarriers [43][44][45][46][47]. A summary of the published works, exploiting small-angle X-ray scattering (SAXS) analyses of liquid crystalline nanostructures and nanoparticles, is presented in Table 1 below.
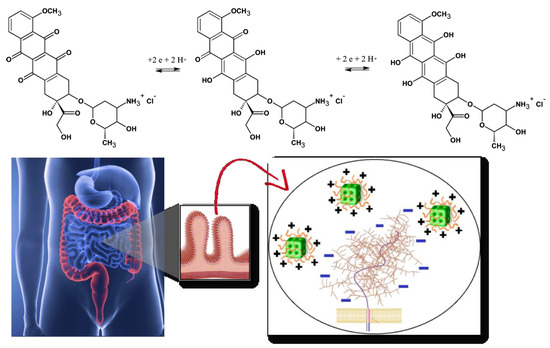
Figure 1. Chemical structures and redox process of the anticancer drug doxorubicin, from left to right: initial, oxidized and reduced forms of doxorubicin (top panel). Scheme of oral drug administration indicating the microvilli of the intestinal membrane, for which bioadhesive positively charged cubosome particles (modified by polyelectrolyte shells) interact with the negative charges of the mucin layer over the mucosal membrane (bottom panel). Either the biocompatible polyelectrolyte shells or the lipids, constituting the cubosome nanocarriers, are pH-sensitive.
Table 1. Summary of reported compositions and main characteristics of self-assembled pH-responsive liquid crystalline mesophases and nanoparticles derived thereof.
| Lipids | Additives | Preparation Methodology | Studied pH Values | Liquid Crystalline Phases | Perspective for Application | Refs. |
|---|---|---|---|---|---|---|
| Monoolein Oleic acid | Brucea javanica oil Pluronic F127 PBS Doxorubicin |
Melting 60 °C Stirring High-pressure homogenization |
7.4 6.8 5.3 |
HII Pn3m, Im3m microemulsion |
Dual-drug (BJO, DOX) delivery/cancer inhibition (in vitro tested) |
[48] |
| Monoolein Oleic acid |
Pluronic F127 PBS |
Heating 80 °C Homogenization High pressure |
6.0 7.0 |
HII Im3m |
Drug delivery (perspective) | [49] |
| Monolinolein Linoleic acid |
Phloroglucinol | Hydration Heating Vortex mixing |
2.0 7.0 |
HII Im3m |
Oral drug delivery (perspective) | [50] |
| Monolinolein Pyridinylmethyl linoleate |
Doxorubicin | Hydration Heating Vortex mixing |
5.5 7.4 |
Pn3m HII |
Tumor-targeted delivery (in vitro tested) |
[51] |
| Monoolein 2-hydroxyoleic Acid |
Pluronic F127 PBS |
Ultrasonication | 2.0; 3.0 3.5; 4.0; 4.5 5.0; 6.0; 7.4 |
Pn3m, HII Pn3m, Im3m Lamellar |
Tumor-targeted delivery (perspective) | [52] |
| Monoolein Phytantriol “Lipid 1” |
Doxorubicin | Melting Hydration Centrifugation |
5.8 7.5 9.0 |
Pn3m Pn3m Pn3m |
Drug delivery (perspective) | [53] |
| Monoolein DOPS |
- | Hydration Vortex mixing Centrifugation |
6.7 2.75 2.55 |
L HII Im3m |
Drug delivery (perspective) | [54] |
| Monoolein N-Oleoyl-glycine N-(2-aminoethyl)-oleamide |
Doxorubicin | Melting Hydration Centrifuge mixing |
5.5 7.5 |
Pn3m Pn3m |
Drug delivery (perspective) | [55] |
| Monoolein Oleic acid Vaccenic acid Gondoic acid Erucic acid Nervonic acid |
Pluronic F127 PBS |
Hydration Ultrasonication |
4.9 7.0 |
Fd3m HII |
Drug delivery (perspective) |
[56] |
| Monoolein “Lipid 3” |
Methylene green zinc chloride double salt | Hydration Centrifugation |
2.5 3.0 5.0 7.0 |
Pn3m Pn3m Pn3m Pn3m |
Drug delivery (perspective) | [57] |
| Monoolein | Nicergoline Pluronic F108 |
Ultrasonication | 3.3; 5.6; 5.9; 6.7 7.2 8.4 |
Im3m Im3m Pn3m, Im3m Pn3m, HII |
Drug delivery (perspective) | [58] |
| Monolinolein | “Outer membrane protein F” |
Heating 45 °C Vortex mixing |
4.8 7.4 |
Pn3m Pn3m |
Drug delivery (perspective) | [59] |
| Monoolein Monolinolein |
Bupivacaine Caprylic acid Capric acid |
Heating 50 °C Hydration Heating 60 °C Vortex mixing Incubation at 37 °C (1–2 weeks) |
6.0 7.4 |
Pn3m HII |
Drug delivery (perspective) | [60] |
| Phytantriol | Pluronic F127 Decyl betainate chloride |
Ultrasonication | 3.9; 5.5 7.4; 8.5 |
Pn3m, L Im3m, HII |
Oral drug delivery (perspective) | [61] |
| DOPE | DNA N,N-dimethyldodecyl- amine-N-oxide |
Hydration Vortex mixing Freeze–thaw |
7.2 4.8 |
HII, L, Pn3m HII, L |
Genetic and drug delivery (perspective) |
[62] |
| Monoolein | PP50 1 Pluronic F127 |
Hydration Sonication Stabilization with surfactant |
7.5 5.5 |
Im3m Im3m, swollen |
Drug delivery (perspective) | [63] |
| Monoolein Phytantriol |
Poloxamer P407 PDMAEMA-b- PLMA |
Hydration Ultrasonication |
4.2 6.0 7.4 |
Im3m, L Im3m, L Im3m, L |
Drug delivery (perspective) | [64] |
| Monoolein | Aspartic acid-leucine peptide Poly-lysine FITC–dextran |
Melting 65 °C Hydration |
3.0; 5.0; 7.0; 8.5 |
Not identified | Drug delivery (perspective) | [65] |
| Monoolein | Modified alginate Modified silk fibroin FITC–dextran |
Melting 60 °C Hydration |
3.0; 4.0; 4.5; 5.0; 7.0; 9.0 |
Not identified | Drug delivery (perspective) | [66] |
| DMPC DMPE |
N,N-dimethyl- dodecylamine- N-oxide Poly(acrylic acid) |
Hydration Repeated heating 60 °C, vortex mixing, ice bath cooling |
<2 3.8 6.8 9.8 |
L (swollen) L (swollen + collap) L (collap + multiL) Im3m, L (collap) |
Therapeutic agent (perspective) |
[67] |
1 PP50: poly(L-lysine-iso-phthalimide) chain grafted with L-phenylalanine.
Oral delivery of peptides, recombinant proteins and other nanomedicines is of a primary therapeutic interest [68][69][70][71][72]. The oral drug administration represents several challenges. One of the most prominent is the considerable pH variation in the gastrointestinal tract (Figure 1, bottom panel). With this concern in mind, we discuss the perspectives of cubosomes development in oral drug delivery applications with special attention to composite nanocarriers, i.e., cubosomes with pH-responsive characteristics provided by polyelectrolyte shells [46][47][72]. The latter may ensure the structural stability of the carriers in adverse media, for instance under the strong acidic condition of the stomach.
Increased oral drug bioavailability can be expected due to the mucoadhesive features of the cubic-phase forming lipids. Prolonged-release mucoadhesive formulations have been obtained thanks to the fusogenic properties of monoolein enabling permeation enhancer activity of the nanocarriers [1][17][18][19]. The mucoadhesive controlled release formulations interact with the mucosal components such as mucin (Figure 1, bottom panel). The enhanced adsorption on the intestinal epithelia ideally promotes a sustained drug release.
Salentinig et al. have emphasized that the hydrolysis of monoolein in the zones of the gastrointestinal tract will produce oleic acid [37], the ionization state of which determines the structural properties of the carriers, through the lipid packing, and hence their fusion dynamics with the membranes [20].
pH-responsive lyotropic liquid crystalline phases and nanoparticles have received tremendous interest in view of applications in anticancer nanomedicine [3][4][11][18][28][29][31]. Although the pH of normal tissues and blood is around 7.4, the tumor environment exhibits acidic pH owing to the metabolic production of lactic acid under the conditions of fast cell growth and deficiency of oxygen and nutrients. The use of pH-sensitive cubosomes as drug delivery systems for cancer treatments presents an advantage of the pH difference between the tumor environment and the normal physiological condition [73][74],. In chemotherapy, pH-responsive drug delivery nanocarriers have been shown to accumulate in the tumor tissue via the enhanced permeability and retention (EPR) effect [73]. The release of anticancer drugs may be triggered in response to extracellular or intracellular chemical stimuli including pH-stimuli [73].
References
- Boyd, B.J.; Khoo, S.; Whittaker, D.; Davey, G.; Porter, C.J. A lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water soluble drug in rats. Int. J. Pharm. 2007, 340, 52–60.
- Tiberg, F.; Johnsson, M. Drug delivery applications of non-lamellar liquid crystalline phases and nanoparticles. J. Drug Deliv. Sci. Technol. 2011, 21, 101–109.
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206.
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.; Li, Y.; Zou, A.-H. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv. Colloid Interface Sci. 2017, 249, 331–345.
- Yaghmur, A.; Tran, B.V.; Moghimi, S.M. Non-Lamellar Liquid Crystalline Nanocarriers for Thymoquinone Encapsulation. Molecules 2019, 25, 16.
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Liquid Crystalline Nanostructures as PEGylated Reservoirs of Omega-3 Polyunsaturated Fatty Acids: Structural Insights toward Delivery Formulations against Neurodegenerative Disorders. ACS Omega 2018, 3, 3235–3247.
- Tan, A.; Hong, L.; Du, J.D.; Boyd, B.J. Self-Assembled Nanostructured Lipid Systems: Is There a Link between Structure and Cytotoxicity? Adv. Sci. 2018, 6, 1801223.
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48.
- Kim, I.-Y.; Kang, Y.-S.; Lee, D.S.; Park, H.-J.; Choi, E.-K.; Oh, Y.-K.; Son, H.-J.; Kim, J.-S. Antitumor activity of EGFR targeted pH-sensitive immunoliposomes encapsulating gemcitabine in A549 xenograft nude mice. J. Control. Release 2009, 140, 55–60.
- Masotti, A. Niosomes as candidate bioconjugates for imaging and pH-sensitive drug delivery nanocarriers for rare pediatric tumors. J. Drug Deliv. Sci. Technol. 2013, 23, 22–24.
- Faria, A.R.; Silvestre, O.; Maibohm, C.; Adão, R.M.R.; Silva, B.F.B.; Nieder, J.B. Cubosome nanoparticles for enhanced delivery of mitochondria anticancer drug elesclomol and therapeutic monitoring via sub-cellular NAD(P)H multi-photon fluorescence lifetime imaging. Nano Res. 2018, 12, 991–998.
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin- and Fish Oil-Loaded Spongosome and Cubosome Nanoparticles with Neuroprotective Potential against H2O2-Induced Oxidative Stress in Differentiated Human SH-SY5Y Cells. ACS Omega 2019, 4, 3061–3073.
- Nithya, R.; Jerold, P.; Siram, K. Cubosomes of dapsone enhanced permeation across the skin. J. Drug Deliv. Sci. Technol. 2018, 48, 75–81.
- Akbar, S.; Anwar, A.; Ayish, A.; Elliott, J.; Squires, A.M. Phytantriol based smart nano-carriers for drug delivery applications. Eur. J. Pharm. Sci. 2017, 101, 31–42.
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67.
- Salah, S.; Mahmoud, A.A.; Kamel, A.O. Etodolac transdermal cubosomes for the treatment of rheumatoid arthritis: Ex vivo permeation and in vivo pharmacokinetic studies. Drug Deliv. 2017, 24, 846–856.
- Chen, J.; Zeng, N.; Gao, X.; Hu, Q.; Song, Q.; Xia, H.; Liu, Z.; Gu, G.; Pang, Z.; Chen, H.; et al. Lipid-based liquid crystalline nanoparticles as oral drug delivery vehicles for poorly water-soluble drugs: Cellular interaction and in vivo absorption. Int. J. Nanomed. 2012, 7, 3703–3718.
- Swarnakar, N.K.; Thanki, K.; Jain, S. Bicontinuous Cubic Liquid Crystalline Nanoparticles for Oral Delivery of Doxorubicin: Implications on Bioavailability, Therapeutic Efficacy, and Cardiotoxicity. Pharm. Res. 2013, 31, 1219–1238.
- Lai, J.; Chen, J.; Lu, Y.; Sun, J.; Hu, F.; Yin, Z.; Wu, W. Glyceryl Monooleate/Poloxamer 407 Cubic Nanoparticles as Oral Drug Delivery Systems: I. In Vitro Evaluation and Enhanced Oral Bioavailability of the Poorly Water-Soluble Drug Simvastatin. AAPS PharmSciTech 2009, 10, 960–966.
- Brendan Dyett; Haitao Yu; Jamie Strachan; Calum J. Drummond; Charlotte E. Conn; Fusion dynamics of cubosome nanocarriers with model cell membranes.. Nature Communications 2019, 10, 1-13, 10.1038/s41467-019-12508-8.
- Angelova, A.; Angelov, B.; Mutafchieva, R.; Lesieur, S.; Couvreur, P.; Patrick, C. Self-Assembled Multicompartment Liquid Crystalline Lipid Carriers for Protein, Peptide, and Nucleic Acid Drug Delivery. Accounts Chem. Res. 2011, 44, 147–156.
- Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Angelova, A. Cubic Liquid Crystalline Nanostructures Involving Catalase and Curcumin: BioSAXS Study and Catalase Peroxidatic Function after Cubosomal Nanoparticle Treatment of Differentiated SH-SY5Y Cells. Molecules 2019, 24, 3058.
- Valldeperas, M.; Salis, A.; Barauskas, J.; Tiberg, F.; Arnebrant, T.; Razumas, V.; Monduzzi, M.; Nylander, T. Enzyme encapsulation in nanostructured self-assembled structures: Toward biofunctional supramolecular assemblies. Curr. Opin. Colloid Interface Sci. 2019, 44, 130–142.
- Angelov, B.; Angelova, A.; Filippov, S.K.; Drechsler, M.; Stepanek, P.; Lesieur, S. Multicompartment Lipid Cubic Nanoparticles with High Protein Upload: Millisecond Dynamics of Formation. ACS Nano 2014, 8, 5216–5226.
- Clogston, J.D.; Caffrey, M. Controlling release from the lipidic cubic phase. Amino acids, peptides, proteins and nucleic acids. J. Control. Release 2005, 107, 97–111.
- Zabara, A.; Mezzenga, R. Controlling molecular transport and sustained drug release in lipid-based liquid crystalline mesophases. J. Control. Release 2014, 188, 31–43.
- Wye-Khay Fong; Tracey Hanley; Ben J. Boyd; Stimuli responsive liquid crystals provide ‘on-demand’ drug delivery in vitro and in vivo. Journal of Controlled Release 2009, 135, 218-226, 10.1016/j.jconrel.2009.01.009.
- Fong, W.-K.; Negrini, R.; Vallooran, J.J.; Mezzenga, R.; Boyd, B.J. Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics. J. Colloid Interface Sci. 2016, 484, 320–339.
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978.
- Fong, W.-K.; Hanley, T.L.; Thierry, B.; Tilley, A.; Kirby, N.; Waddington, L.J.; Boyd, B.J. Understanding the photothermal heating effect in non-lamellar liquid crystalline systems, and the design of new mixed lipid systems for photothermal on-demand drug delivery. Phys. Chem. Chem. Phys. 2014, 16, 24936–24953.
- Rarokar, N.; Saoji, S.; Raut, N.A.; Taksande, J.B.; Khedekar, P.B.; Dave, V.S. Nanostructured Cubosomes in a Thermoresponsive Depot System: An Alternative Approach for the Controlled Delivery of Docetaxel. AAPS PharmSciTech 2015, 17, 436–445.
- Liu, Q.; Dong, Y.-D.; Hanley, T.L.; Boyd, B.J. Sensitivity of Nanostructure in Charged Cubosomes to Phase Changes Triggered by Ionic Species in Solution. Langmuir 2013, 29, 14265–14273.
- Muir, B.W.; Zhen, G.; Gunatillake, P.; Hartley, P. Salt Induced Lamellar to Bicontinuous Cubic Phase Transitions in Cationic Nanoparticles. J. Phys. Chem. B 2012, 116, 3551–3556.
- Barriga, H.M.G.; Tyler, A.; McCarthy, N.L.C.; Parsons, E.S.; Ces, O.; Law, R.; Seddon, J.M.; Brooks, N.J. Temperature and pressure tuneable swollen bicontinuous cubic phases approaching nature’s length scales. Soft Matter 2015, 11, 600–607.
- Borné, J.; Nylander, T.; Khan, A. Phase Behavior and Aggregate Formation for the Aqueous Monoolein System Mixed with Sodium Oleate and Oleic Acid. Langmuir 2001, 17, 7742–7751.
- Mele, S.; Söderman, O.; Ljusberg-Wahrén, H.; Thuresson, K.; Monduzzi, M.; Nylander, T. Phase behavior in the biologically important oleic acid/sodium oleate/water system. Chem. Phys. Lipids 2018, 211, 30–36.
- Salentinig, S.; Sagalowicz, L.; Glatter, O. Self-Assembled Structures and pKaValue of Oleic Acid in Systems of Biological Relevance. Langmuir 2010, 26, 11670–11679.
- Yaghmur, A.; Sartori, B.; Rappolt, M. Self-Assembled Nanostructures of Fully Hydrated Monoelaidin–Elaidic Acid and Monoelaidin–Oleic Acid Systems. Langmuir 2012, 28, 10105–10119.
- Gontsarik, M.; Mohammadtaheri, M.; Yaghmur, A.; Salentinig, S. pH-Triggered nanostructural transformations in antimicrobial peptide/oleic acid self-assemblies. Biomater. Sci. 2018, 6, 803–812.
- Tran, N.; Hawley, A.M.; Hinton, T.M.; Mudie, S.T.; Giakoumatos, E.C.; Waddington, L.J.; Kirby, N.; Drummond, C.J.; Mulet, X.; Muir, B.W. Nanostructure and cytotoxicity of self-assembled monoolein–capric acid lyotropic liquid crystalline nanoparticles. RSC Adv. 2015, 5, 26785–26795.
- Tran, N.; Mulet, X.; Hawley, A.M.; Fong, C.; Zhai, J.; Le, T.C.; Ratcliffe, J.; Drummond, C.J. Manipulating the Ordered Nanostructure of Self-Assembled Monoolein and Phytantriol Nanoparticles with Unsaturated Fatty Acids. Langmuir 2018, 34, 2764–2773.
- Tran, N.; Hawley, A.M.; Zhai, J.; Muir, B.W.; Fong, C.; Drummond, C.J.; Mulet, X. High-Throughput Screening of Saturated Fatty Acid Influence on Nanostructure of Lyotropic Liquid Crystalline Lipid Nanoparticles. Langmuir 2016, 32, 4509–4520.
- Wei, Y.; Zhang, J.; Zheng, Y.; Gong, Y.; Fu, M.; Liu, C.; Xu, L.; Sun, C.C.; Gao, Y.; Qian, S. Cubosomes with surface cross-linked chitosan exhibit sustained release and bioavailability enhancement for vinpocetine. RSC Adv. 2019, 9, 6287–6298.
- Verma, P.; Ahuja, M. Optimization, characterization and evaluation of chitosan-tailored cubic nanoparticles of clotrimazole. Int. J. Boil. Macromol. 2015, 73, 138–145.
- Svensson, O.; Thuresson, K.; Arnebrant, T. Interactions between chitosan-modified particles and mucin-coated surfaces. J. Colloid Interface Sci. 2008, 325, 346–350.
- Mathews, P.D.; Mertins, O. Dispersion of chitosan in liquid crystalline lamellar phase: Production of biofriendly hydrogel of nano cubic topology. Carbohydr. Polym. 2017, 157, 850–857.
- Mathews, P.D.; Patta, A.C.M.F.; Gonçalves, J.V.; Gama, G.D.S.; Garcia, I.T.S.; Mertins, O. Targeted Drug Delivery and Treatment of Endoparasites with Biocompatible Particles of pH-Responsive Structure. Biomacromolecules 2018, 19, 499–510.
- Yawen Li; Angelina Angelova; Fangzhou Hu; V. M. Garamus; Changjun Peng; Na Li; Jianwen Liu; Dan Liu; Aihua Zou; pH Responsiveness of Hexosomes and Cubosomes for Combined Delivery of Brucea javanica Oil and Doxorubicin. Langmuir 2019, 35, 14532-14542, 10.1021/acs.langmuir.9b02257.
- Minoru Nakano; Takashi Teshigawara; Atsuhiko Sugita; Warunee Leesajakul; Atsuhiko Taniguchi; Tomoari Kamo; Hideki Matsuoka; Tetsurou Handa; Dispersions of Liquid Crystalline Phases of the Monoolein/Oleic Acid/Pluronic F127 System. Langmuir 2002, 18, 9283-9288, 10.1021/la026297r.
- Renata Negrini; Raffaele Mezzenga; pH-Responsive Lyotropic Liquid Crystals for Controlled Drug Delivery. Langmuir 2011, 27, 5296-5303, 10.1021/la200591u.
- Renata Negrini; Raffaele Mezzenga; Wye-Khay Fong; Ben J. Boyd; pH-responsive lyotropic liquid crystals and their potential therapeutic role in cancer treatment. Chemical Communications 2015, 51, 6671-6674, 10.1039/C4CC10274F.
- Rama Prajapati; Mark Gontsarik; Anan Yaghmur; Stefan Salentinig; pH-Responsive Nano-Self-Assemblies of the Anticancer Drug 2-Hydroxyoleic Acid. Langmuir 2019, 35, 7954-7961, 10.1021/acs.langmuir.9b00838.
- Ewa Nazaruk; Monika Szlęzak; Ewa Gorecka; Renata Bilewicz; Yazmin M. Osornio; Peter Uebelhart; Ehud M. Landau; Design and Assembly of pH-Sensitive Lipidic Cubic Phase Matrices for Drug Release. Langmuir 2014, 30, 1383-1390, 10.1021/la403694e.
- Toshihiko Oka; Moynul Hasan; Zahidul Islam; Moniruzzaman; Masahito Yamazaki; Low-pH-Induced Lamellar to Bicontinuous Primitive Cubic Phase Transition in Dioleoylphosphatidylserine/Monoolein Membranes. Langmuir 2017, 33, 12487-12496, 10.1021/acs.langmuir.7b02512.
- Ewa Nazaruk; Ewa Gorecka; Yazmin M. Osornio; Ehud M. Landau; Renata Bilewicz; Charged additives modify drug release rates from lipidic cubic phase carriers by modulating electrostatic interactions. Journal of Electroanalytical Chemistry 2018, 819, 269-274, 10.1016/j.jelechem.2017.10.057.
- Celesta Fong; Jiali Zhai; Calum J. Drummond; Nhiem Tran; Micellar Fd3m cubosomes from monoolein – long chain unsaturated fatty acid mixtures: Stability on temperature and pH response. Journal of Colloid and Interface Science 2020, 566, 98-106, 10.1016/j.jcis.2020.01.041.
- Nelli Rahanyan-Kägi; Simone Aleandri; Chiara Speziale; Raffaele Mezzenga; Ehud M. Landau; Stimuli-Responsive Lipidic Cubic Phase: Triggered Release and Sequestration of Guest Molecules. Chemistry – A European Journal 2014, 21, 1873-1877, 10.1002/chem.201405580.
- Stefan Salentinig; Kristian J. Tangso; Adrian Hawley; Ben J. Boyd; pH-Driven Colloidal Transformations Based on the Vasoactive Drug Nicergoline. Langmuir 2014, 30, 14776-14781, 10.1021/la503824z.
- Alexandru Zabara; Renata Negrini; Ozana Onaca-Fischer; Raffaele Mezzenga; Perforated Bicontinuous Cubic Phases with pH-Responsive Topological Channel Interconnectivity. Small 2013, 9, 3602-3609, 10.1002/smll.201300348.
- Anan Yaghmur; Michael Rappolt; Jesper Østergaard; Claus Larsen; Susan Larsen; Characterization of Bupivacaine-Loaded Formulations Based on Liquid Crystalline phases and Microemulsions: The Effect of Lipid Composition. Langmuir 2012, 28, 2881-2889, 10.1021/la203577v.
- Iris R. Ribeiro; Maira F. Immich; Dan Lundberg; Fernanda Poletto; Watson Loh; Physiological neutral pH drives a gradual lamellar-to-reverse cubic-to-reverse hexagonal phase transition in phytantriol-based nanoparticles. Colloids and Surfaces B: Biointerfaces 2019, 177, 204-210, 10.1016/j.colsurfb.2019.01.055.
- Lukáš Hubčík; Sérgio S. Funari; Petra Pullmannová; Ferdinand Devínsky; Daniela Uhrikova; Stimuli responsive polymorphism of C12NO/DOPE/DNA complexes: Effect of pH, temperature and composition. Biochimica et Biophysica Acta (BBA) - Biomembranes 2015, 1848, 1127-1138, 10.1016/j.bbamem.2015.01.020.
- Monika Kluzek; Shiqi Wang; Rongjun Chen; Carlos M Marques; Fabrice Thalmann; John Seddon; Marc Schmutz; Arwen Tyler; Influence of a pH-sensitive polymer on the structure of monoolein cubosomes. Soft Matter 2017, 13, 7571-7577, 10.1039/C7SM01620D.
- Chountoulesi, M.; Pippa, N.; Chrysostomou, V.; Pispas, S.; Chrysina, E.D.; Forys, A.; Otulakowski, L.; Trzebicka, B.; Demetzos, C.; Stimuli-Responsive Lyotropic Liquid Crystalline Nanosystems with Incorporated Poly(2-Dimethylamino Ethyl Methacrylate)-b-Poly(Lauryl Methacrylate) Amphiphilic Block Copolymer. Polymers 2019, 11, 1400.
- Hee Jin Seo; Jin-Chul Kim; Controlled Release from Monoolein Cubic Phase by Complexation Between Acidic Proteinoid and Basic Proteinoid. Journal of Dispersion Science and Technology 2012, 33, 605-610, 10.1080/02726351.2011.574891.
- Taek Kwan Kwon; Jin-Chul Kim; Complex Coacervation-Controlled Release from Monoolein Cubic Phase Containing Silk Fibroin and Alginate. Biomacromolecules 2011, 12, 466-471, 10.1021/bm101249e.
- Crisci, A.; Hay, D.N.T.; Seifert, S.; Firestone, M.A.; pH- and Ionic-Strength-Induced Structural Changes in Poly(acrylic acid)-Lipid-Based Self-Assembled Materials. Macromol. Symp. 2009, 281, 126–134.
- Li, P.; Nielsen, H.M.; Müllertz, A. Oral delivery of peptides and proteins using lipid-based drug delivery systems. Expert Opin. Drug Deliv. 2012, 9, 1289–1304.
- Shah, M.H.; Paradkar, A. Cubic liquid crystalline glyceryl monooleate matrices for oral delivery of enzyme. Int. J. Pharm. 2005, 294, 161–171.
- He, S.; Liu, Z.; Xu, D. Advance in oral delivery systems for therapeutic protein. J. Drug Target. 2018, 27, 283–291.
- Smart, A.L.; Gaisford, S.; Basit, A.W. Oral peptide and protein delivery: Intestinal obstacles and commercial prospects. Expert Opin. Drug Deliv. 2014, 11, 1323–1335.
- Patta, A.C.F.; Mathews, P.D.; Madrid, R.R.; Rigoni, V.L.; Silva, E.R.; Mertins, O. Polyionic complexes of chitosan-N-arginine with alginate as pH responsive and mucoadhesive particles for oral drug delivery applications. Int. J. Boil. Macromol. 2020, 148, 550–564.
- Tannock, I.F.; Rotin, D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989, 49, 4373–4384.
- Manchun, S.; Dass, C.R.; Sriamornsak, P. Targeted therapy for cancer using pH-responsive nanocarrier systems. Life Sci. 2012, 90, 381–387.




