Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cubosomes are soft biocontinuous nanoparticles whose 3D geometry can be engineered to render the structures responsive to pH variations, which is of large interest to the production of efficient drug delivery materials. We have reviewed the literature to provide a state of the art in this regard and shed lights on prominent perspectives and strategies for pH-sensitive cubosomes development, taking advantage of the pH changes of the biological media at targeted application sites.
- Lipids membrane
- Polyelectrolites
- Nanocarriers
- Drug release
- Cell interaction
- Doxorubicin
- Cubosomes
Lipid nanoparticle-mediated drug delivery experiences rapid developments in the field of liquid crystalline colloidal carriers, e.g., cubosomes, spongosomes, hexosomes and vesicles [1,2,3,4,5,6,7]. In addition to the expansion of liposomes as advanced drug delivery systems [8,9,10], a plethora of research has been dedicated to diverse mesoporous liquid crystalline materials and nanostructures intended as drug delivery devices [11,12,13,14,15,16]. Lipid-based cubic mesophases are increasingly implemented in noninvasive drug delivery applications [17,18,19]. The liquid crystalline structure of cubosomes, consisting of well-defined networks of aqueous channels and lipid bilayer membranes, organized in periodic 3D topologies, presents advantages over other delivery systems [20]. Scientific and technological advances have been achieved in the fabrication of biocompatible systems for encapsulation of natural and synthetic lipophilic, hydrophilic and hydrophobic drug molecules, a variety of macromolecular drugs (peptides, proteins, DNA, siRNA, etc.) and imaging agents [1,2,3,4,5,6,7,11,12,13,14,15,16,17,18,19,21,22,23,24]. Complex cubic lattice networks of high surface areas provide enhanced protection of the incorporated payload from degradation as well as the prolonged and sustained release of the entrapped bioactive molecules [25,26].
Current demands for improved performance and specificity of drug delivery carriers require the use of intelligent materials, which respond to various environmental stimuli [27]. In this context, the cubosome assemblies present further advantages because the transformations between the different liquid crystalline organizations, e.g., Pn3m, Im3m and Ia3d, besides the inverted hexagonal phases (Figure 1), can be tuned and controlled by changes in temperature, ionic strength or pH of the environment of the targeted application sites [28,29,30,31,32,33,34].
In the present work, we review recent advances in cubosome nanocarriers and bulk cubic mesophases with a particular emphasis on the pH effects on the structures and topologies of the designed drug delivery systems. This interest is motivated by the fact that pH represents an inherent physiological condition of all biological organisms and that pathological (inflamed or infected) areas can embed target sites for pH-sensitive nanomedicines. This has led to the emergence of pH-responsive drug delivery carriers and nanomaterials, some of which are reviewed here below.
Acidic compartments of the cells are the endosomes (pH 5–6) and the lysosomes (pH 5). Depending on the application site, pH changes of the biological medium may comprise a profitable condition to boost the target release of encapsulated drugs from the pH-responsive nanocarriers. In this context, lipid-based pH-sensitive cubosomes have been produced by the assembly of traditional cubic-phase-forming amphiphiles monoolein or phytantriol with charged or ionizable lipids [35,36,37,38,39,40,41,42]. Incorporation of drugs like doxorubicin, for which the redox process (Figure 1) provokes pH-dependent structural changes, may also lead to pH-responsiveness of the host cubic phase carriers. Furthermore, the association of lipids with polyelectrolytes and charged surfactants was employed as a means to generate highly pH-sensitive cubosomes and other types of nanocarriers [43,44,45,46,47]. A summary of the published works, exploiting small-angle X-ray scattering (SAXS) analyses of liquid crystalline nanostructures and nanoparticles, is presented in Table 1 below.
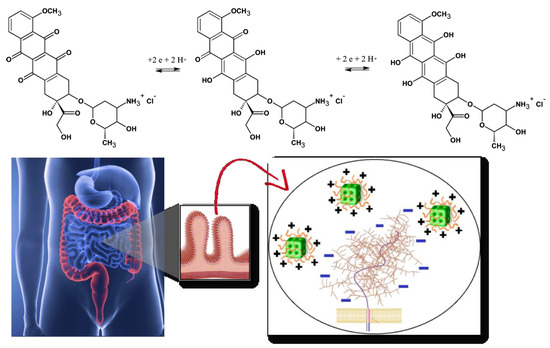
Figure 1. Chemical structures and redox process of the anticancer drug doxorubicin, from left to right: initial, oxidized and reduced forms of doxorubicin (top panel). Scheme of oral drug administration indicating the microvilli of the intestinal membrane, for which bioadhesive positively charged cubosome particles (modified by polyelectrolyte shells) interact with the negative charges of the mucin layer over the mucosal membrane (bottom panel). Either the biocompatible polyelectrolyte shells or the lipids, constituting the cubosome nanocarriers, are pH-sensitive.
Table 1. Summary of reported compositions and main characteristics of self-assembled pH-responsive liquid crystalline mesophases and nanoparticles derived thereof.
| Lipids | Additives | Preparation Methodology | Studied pH Values | Liquid Crystalline Phases | Perspective for Application | Refs. |
|---|---|---|---|---|---|---|
| Monoolein Oleic acid | Brucea javanica oil Pluronic F127 PBS Doxorubicin |
Melting 60 °C Stirring High-pressure homogenization |
7.4 6.8 5.3 |
HII Pn3m, Im3m microemulsion |
Dual-drug (BJO, DOX) delivery/cancer inhibition (in vitro tested) |
[59] |
| Monoolein Oleic acid |
Pluronic F127 PBS |
Heating 80 °C Homogenization High pressure |
6.0 7.0 |
HII Im3m |
Drug delivery (perspective) | [60] |
| Monolinolein Linoleic acid |
Phloroglucinol | Hydration Heating Vortex mixing |
2.0 7.0 |
HII Im3m |
Oral drug delivery (perspective) | [61] |
| Monolinolein Pyridinylmethyl linoleate |
Doxorubicin | Hydration Heating Vortex mixing |
5.5 7.4 |
Pn3m HII |
Tumor-targeted delivery (in vitro tested) |
[62] |
| Monoolein 2-hydroxyoleic Acid |
Pluronic F127 PBS |
Ultrasonication | 2.0; 3.0 3.5; 4.0; 4.5 5.0; 6.0; 7.4 |
Pn3m, HII Pn3m, Im3m Lamellar |
Tumor-targeted delivery (perspective) | [63] |
| Monoolein Phytantriol “Lipid 1” |
Doxorubicin | Melting Hydration Centrifugation |
5.8 7.5 9.0 |
Pn3m Pn3m Pn3m |
Drug delivery (perspective) | [64] |
| Monoolein DOPS |
- | Hydration Vortex mixing Centrifugation |
6.7 2.75 2.55 |
L HII Im3m |
Drug delivery (perspective) | [66] |
| Monoolein N-Oleoyl-glycine N-(2-aminoethyl)-oleamide |
Doxorubicin | Melting Hydration Centrifuge mixing |
5.5 7.5 |
Pn3m Pn3m |
Drug delivery (perspective) | [69] |
| Monoolein Oleic acid Vaccenic acid Gondoic acid Erucic acid Nervonic acid |
Pluronic F127 PBS |
Hydration Ultrasonication |
4.9 7.0 |
Fd3m HII |
Drug delivery (perspective) |
[70] |
| Monoolein “Lipid 3” |
Methylene green zinc chloride double salt | Hydration Centrifugation |
2.5 3.0 5.0 7.0 |
Pn3m Pn3m Pn3m Pn3m |
Drug delivery (perspective) | [71] |
| Monoolein | Nicergoline Pluronic F108 |
Ultrasonication | 3.3; 5.6; 5.9; 6.7 7.2 8.4 |
Im3m Im3m Pn3m, Im3m Pn3m, HII |
Drug delivery (perspective) | [72] |
| Monolinolein | “Outer membrane protein F” |
Heating 45 °C Vortex mixing |
4.8 7.4 |
Pn3m Pn3m |
Drug delivery (perspective) | [73] |
| Monoolein Monolinolein |
Bupivacaine Caprylic acid Capric acid |
Heating 50 °C Hydration Heating 60 °C Vortex mixing Incubation at 37 °C (1–2 weeks) |
6.0 7.4 |
Pn3m HII |
Drug delivery (perspective) | [74] |
| Phytantriol | Pluronic F127 Decyl betainate chloride |
Ultrasonication | 3.9; 5.5 7.4; 8.5 |
Pn3m, L Im3m, HII |
Oral drug delivery (perspective) | [75] |
| DOPE | DNA N,N-dimethyldodecyl- amine-N-oxide |
Hydration Vortex mixing Freeze–thaw |
7.2 4.8 |
HII, L, Pn3m HII, L |
Genetic and drug delivery (perspective) |
[76] |
| Monoolein | PP50 1 Pluronic F127 |
Hydration Sonication Stabilization with surfactant |
7.5 5.5 |
Im3m Im3m, swollen |
Drug delivery (perspective) | [77] |
| Monoolein Phytantriol |
Poloxamer P407 PDMAEMA-b- PLMA |
Hydration Ultrasonication |
4.2 6.0 7.4 |
Im3m, L Im3m, L Im3m, L |
Drug delivery (perspective) | [78] |
| Monoolein | Aspartic acid-leucine peptide Poly-lysine FITC–dextran |
Melting 65 °C Hydration |
3.0; 5.0; 7.0; 8.5 |
Not identified | Drug delivery (perspective) | [79] |
| Monoolein | Modified alginate Modified silk fibroin FITC–dextran |
Melting 60 °C Hydration |
3.0; 4.0; 4.5; 5.0; 7.0; 9.0 |
Not identified | Drug delivery (perspective) | [80] |
| DMPC DMPE |
N,N-dimethyl- dodecylamine- N-oxide Poly(acrylic acid) |
Hydration Repeated heating 60 °C, vortex mixing, ice bath cooling |
<2 3.8 6.8 9.8 |
L (swollen) L (swollen + collap) L (collap + multiL) Im3m, L (collap) |
Therapeutic agent (perspective) |
[81] |
1 PP50: poly(L-lysine-iso-phthalimide) chain grafted with L-phenylalanine.
Oral delivery of peptides, recombinant proteins and other nanomedicines is of a primary therapeutic interest [48,49,50,51,52]. The oral drug administration represents several challenges. One of the most prominent is the considerable pH variation in the gastrointestinal tract (Figure 1, bottom panel). With this concern in mind, we discuss the perspectives of cubosomes development in oral drug delivery applications with special attention to composite nanocarriers, i.e., cubosomes with pH-responsive characteristics provided by polyelectrolyte shells [46,47,52]. The latter may ensure the structural stability of the carriers in adverse media, for instance under the strong acidic condition of the stomach.
Increased oral drug bioavailability can be expected due to the mucoadhesive features of the cubic-phase forming lipids. Prolonged-release mucoadhesive formulations have been obtained thanks to the fusogenic properties of monoolein enabling permeation enhancer activity of the nanocarriers [1,17,18,19]. The mucoadhesive controlled release formulations interact with the mucosal components such as mucin (Figure 1, bottom panel). The enhanced adsorption on the intestinal epithelia ideally promotes a sustained drug release.
Salentinig et al. have emphasized that the hydrolysis of monoolein in the zones of the gastrointestinal tract will produce oleic acid [37], the ionization state of which determines the structural properties of the carriers, through the lipid packing, and hence their fusion dynamics with the membranes [20].
pH-responsive lyotropic liquid crystalline phases and nanoparticles have received tremendous interest in view of applications in anticancer nanomedicine [3,4,11,18,28,29,31]. Although the pH of normal tissues and blood is around 7.4, the tumor environment exhibits acidic pH owing to the metabolic production of lactic acid under the conditions of fast cell growth and deficiency of oxygen and nutrients. The use of pH-sensitive cubosomes as drug delivery systems for cancer treatments presents an advantage of the pH difference between the tumor environment and the normal physiological condition [53,54]. In chemotherapy, pH-responsive drug delivery nanocarriers have been shown to accumulate in the tumor tissue via the enhanced permeability and retention (EPR) effect [53]. The release of anticancer drugs may be triggered in response to extracellular or intracellular chemical stimuli including pH-stimuli [53].
This entry is adapted from the peer-reviewed paper 10.3390/nano10050963
References
- For the corresponding references, please refer to: doi: 10.3390/nano10050963
This entry is offline, you can click here to edit this entry!
