
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chiara Castellini | + 5553 word(s) | 5553 | 2021-05-12 11:41:50 | | | |
| 2 | Lily Guo | -2149 word(s) | 3404 | 2021-06-01 06:10:31 | | |
Video Upload Options
The dogma of mitochondria as the major source of energy in supporting sperm motility should be critically reconsidered in the light of several experimental data pointing to a major role of glycolysis in mammalian spermatozoa. In this light, the reported positive correlation between the mitochondrial membrane potential (ΔΨm) and motility of ejaculated spermatozoa cannot be explained convincingly by an impaired mitochondrial ATP generation only. Evidence has been produced suggesting that, in human sperm, dysfunctional mitochondria represent the main site of generation of reactive oxygen species (ROS). Furthermore, in these organelles, a complex bidirectional relationship could exist between ROS generation and apoptosis-like events that synergize with oxidative stress in impairing sperm biological integrity and functions. Despite the activity of enzymatic and non-enzymatic antioxidant factors, human spermatozoa are particularly vulnerable to oxidative stress, which plays a major role in male factor infertility.
1. Introduction
metabolism and oxidative/apoptotic events.
2. Are Mitochondria Really the Energetic Motor of Mammalian Sperm?
The role of mitochondria in the energetic support of sperm motility is a matter of debate [11,12,13]. Two pathways can account for the generation of ATP in mammalian spermatozoa, glycolysis and mitochondrial respiration. As mitochondrial OXPHOS is much more efficient than glycolysis in generating ATP, it has been widely accepted that the ATP needed for sperm motility is synthesized by mitochondrial respiration.
In mammalian spermatozoa, mitochondria rearrange in tubular structures that are helically distributed around the anterior portion of the axoneme, constituting the midpiece [14,15]. As the sperm flagellum is long and thin and mitochondria are confined in its proximal end, the question has been raised as to whether OXPHOS-derived ATP can passively diffuse through the entire flagellum to efficiently support axoneme activity. In sea urchin sperm, a shuttle mechanism to facilitate the ATP diffusion along the flagellum is provided by the creatine phosphate (CrP) that buffers the ATP/adenosine diphosphate (ADP) ratio at the expense of CrP/creatine [16]. However, mammalian spermatozoa lack or contain only low amounts of CrP or other phosphagens [17,18], making it unlikely that the CrP shuttle plays a major role in providing ATP from mitochondria to the axoneme. Indeed, spermatozoa from knockout mouse models where the gene for the mitochondrial isotype of creatine kinase had been inactivated exhibited similar motility patterns to the wild-type controls [19]. These legitimate considerations shifted the focus from OXPHOS to glycolysis.
Although mitochondrial respiration is more efficient than glycolysis in generating ATP molecules, key enzymes of glycolysis are tethered to the fibrous sheath of the principal piece [20,21,22,23], and hence they might assure an efficient production of ATP for dynein ATPase locally in the entire length of the flagellum. Consistent with this view, in mouse [3], bovine [24] and human spermatozoa [5,6,7], motility was not affected by mitochondrial inhibition when glucose was available in the extracell ular medium. We previously demonstrated that in a medium lacking glycolysable sugars, the presence of substrates for OXPHOS such as pyruvate and lactate fully supported the motility of human spermatozoa [7].
Interestingly, under such experimental conditions, the addition of 2-Deoxy-D-glucose (DOG), which inhibits glycolysis by competing with glucose for key enzymes, significantly decreased sperm motility [7]. This evidence was incompatible with the hypothesis that ATP is synthesized in mitochondria and then provided to the entire axoneme by diffusion.
On the contrary, these findings supported the notion that ATP produced by OXPHOS is used to drive gluconeogenesis and thus to supply glucose to glycolytic enzymes for ATP production in the principal piece.
In this light, glycolysis would compensate for any lack of ATP production by mitochondria in maintaining sperm motility, and mitochondrial OXPHOS inhibition could depress motility only under experimental conditions of concomitant glycolysis blockage. However, differences among the species exist, as stallion spermatozoa rely primarily on mitochondrial respiration to generate energy required for motility [25]. Overall, it is conceivable that both glycolysis and OXPHOS contribute to ATP production, depending on each other in controlling sperm functions according to the different availability of energetic substrates in the environment [4]. Of note, in female genital tract fluids, the concentrations of lactate are higher than those of glycolysable substrates [26,27,28,29], suggesting a possible major role of mitochondrial respiration in supporting sperm motility. This hypothesis could explain why spermatozoa retain a high number of mitochondria during their differentiation, despite the dramatical decrease in the cellular volume resulting from the removal of any unnecessary structure. Anyway, an obligatory role for glycolysis seems to be confirmed by the loss of progressive motility in spermatozoa of mouse models where the gene for sperm-specific glyceraldehyde-3-phosphate dehydrogenases had been knocked out [4]. In this view, the reported correlation of the mitochondrial membrane potential (∆Ψm) [30] or mitochondrial morphologic integrity [31] with the motility of ejaculated spermatozoa cannot be explained convincingly by an impaired mitochondrial ATP generation only.
Noteworthy, in human spermatozoa, a mitochondrial dysfunction could affect motility when it is accompanied by an intrinsic generation of ROS. Oxidative stress, indeed, is responsible for membrane lipid peroxidation [5,32] and promotes the activation of mitochondrial pathways resulting in apoptosis-like changes.
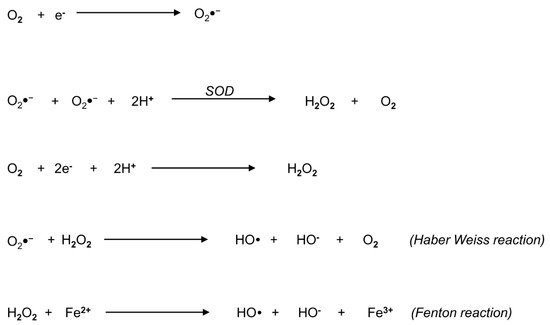
3. Biochemistry of Reactive Oxygen Species: An Overview
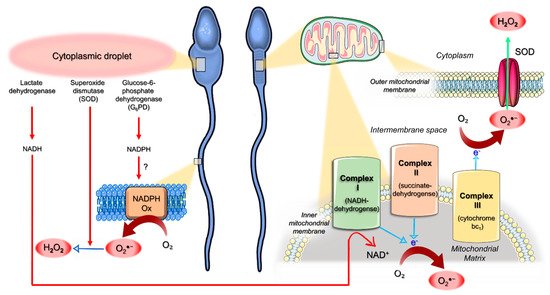
4. Origin of ROS in Semen
5. Mitochondria as an Interplay Center between Oxidative Stress and Apoptotic Events
6. Pathophysiology of Oxidative Stress in Human Spermatozoa
7. Conclusions
References
- Cummins, J. Mitochondrial DNA in mammalian reproduction. Rev. Reprod. 1998, 3, 172–182.
- Saraste, M. Oxidative phosphorylation at the fin de siècle. Science 1999, 283, 1488–1493.
- Mukai, C.; Okuno, M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol. Reprod. 2004, 71, 540–547.
- Miki, K.; Qu, W.; Goulding, E.H.; Willis, W.D.; Bunch, D.O.; Strader, L.F.; Perreault, S.D.; Eddy, E.M.; O’Brien, D.A. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc. Natl. Acad. Sci. USA 2004, 101, 16501–16506.
- Losano, J.D.A.; Padín, J.F.; Méndez-López, I.; Angrimani, D.S.R.; García, A.G.; Barnabe, V.H.; Nichi, M. The Stimulated Glycolytic Pathway Is Able to Maintain ATP Levels and Kinetic Patterns of Bovine Epididymal Sperm Subjected to Mitochondrial Uncoupling. Oxid. Med. Cell Longev. 2017, 1682393.
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207.
- Nascimento, J.M.; Shi, L.Z.; Tam, J.; Chandsawangbhuwana, C.; Durrant, B.; Botvinick, E.L.; Berns, M.W. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J. Cell Physiol. 2008, 217, 745–751.
- Barbonetti, A.; Vassallo, M.R.; Fortunato, D.; Francavilla, S.; Maccarrone, M.; Francavilla, F. Energetic metabolism and human sperm motility: Impact of CB1 receptor activation. Endocrinology 2010, 151, 5882–5892.
- Ortega-Ferrusola, C.; García, B.M.; Gallardo-Bolaños, J.M.; González-Fernández, L.; Rodríguez-Martinez, H.; Tapia, J.A.; Peña, F.J. Apoptotic markers can be used to forecast the freezeability of stallion spermatozoa. Anim. Reprod. Sci. 2009, 114, 393–403.
- Amaral, A.; Paiva, C.; Attardo Parrinello, C.; Estanyol, J.M.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Identification of proteins involved in human sperm motility using high-throughput differential proteomics. J. Proteome Res. 2014, 13, 5670–5684.
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258.
- Aitken, R.J.; Koppers, A.J. Apoptosis and DNA damage in human spermatozoa. Asian J. Androl. 2011, 13, 36–42.
- Plante, M.; de Lamirande, E.; Gagnon, C. Reactive oxygen species released by activated neutrophils, but not by deficient spermatozoa, are sufficient to affect normal sperm motility. Fertil. Steril. 1994, 62, 387–393.
- Micillo, A.; Vassallo, M.R.; Cordeschi, G.; D’Andrea, S.; Necozione, S.; Francavilla, F.; Francavilla, S.; Barbonetti, A. Semen leukocytes and oxidative-dependent DNA damage of spermatozoa in male partners of subfertile couples with no symptoms of genital tract infection. Andrology 2016, 4, 808–815.
- Castellini, C.; D’Andrea, S.; Martorella, A.; Minaldi, E.; Necozione, S.; Francavilla, F.; Francavilla, S.; Barbonetti, A. Relationship between leukocytospermia, reproductive potential after assisted reproductive technology, and sperm parameters: A systematic review and meta-analysis of case-control studies. Andrology 2020, 8, 125–135.
- Agarwal, A.; Salch, R.H.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843.
- Aitken, R.J.; Paterson, M.; Fisher, H.; Buckingham, D.W.; van Duin, M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J. Cell Sci. 1995, 108, 2017–2025.
- Orlando, C.; Krausz, C.; Forti, G.; Casano, R. Simultaneous measurement of sperm LDH, LDH-X, CPK activities and ATP content in normospermic and oligozoospermic men. Int. J. Androl. 1994, 17, 13–18.
- Gomez, E.; Buckingham, D.W.; Brindle, J.; Lanzafame, F.; Irvine, D.S.; Aitken, R.J. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: Correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J. Androl. 1996, 17, 276–287.
- Han, D.; Williams, E.; Cadenas, E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 2001, 353 Pt 2, 411–416.
- Chen, Y.R.; Zweier, J.L. Cardiac mitochondria and reactive oxygen species generation. Circ. Res. 2014, 114, 524–537.
- Han, D.; Antunes, F.; Canali, R.; Rettori, D.; Cadenas, E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J. Biol. Chem. 2003, 278, 5557–5563.
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495.
- Aitken, R.J.; Whiting, S.; De Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060.
- Hotchkiss, R.S.; Strasser, A.; McDunn, J.E.; Swanson, P.E. Cell death. N. Engl. J. Med. 2009, 361, 1570–1583.
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489.
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000, 102, 33–42.
- Goldstein, J.C.; Muñoz-Pinedo, C.; Ricci, J.E.; Adams, S.R.; Kelekar, A.; Schuler, M.; Tsien, R.Y.; Green, D.R. Cytochrome c is released in a single step during apoptosis. Cell Death Differ. 2005, 12, 453–462.
- Wu, H.; Tschopp, J.; Lin, S.C. Smac mimetics and TNFalpha: A dangerous liaison? Cell 2007, 131, 655–658.
- Strasser, A. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 2005, 5, 189–200.
- Cai, J.; Jones, D.P. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J. Biol. Chem. 1998, 273, 11401–11404.
- Barbonetti, A.; Castellini, C.; Di Giammarco, N.; Santilli, G.; Francavilla, S.; Francavilla, F. In vitro exposure of human spermatozoa to bisphenol A induces pro-oxidative/apoptotic mitochondrial dysfunction. Reprod. Toxicol. 2016, 66, 61–67.
- De Lamirande, E.; Gagnon, C. Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic. Biol. Med. 1993, 14, 157–166.
- Aitken, R.J.; Ryan, A.L.; Baker, M.A.; McLaughlin, E.A. Redox activity associated with the maturation and capacitation of mammalian spermatozoa. Free Radic. Biol. Med. 2004, 36, 994–1010.
- De Lamirande, E.; Tsai, C.; Harakat, A.; Gagnon, C. Involvement of reactive oxygen species in human sperm arcosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J. Androl. 1998, 19, 585–594.
- Aitken, R.J.; Buckingham, D.W.; Brindle, J.; Gomez, E.; Baker, H.W.; Irvine, D.S. Analysis of sperm movement in relation to the oxidative stress created by leukocytes in washed sperm preparations and seminal plasma. Hum. Reprod. 1995, 10, 2061–2071.
- Aitken, R.J. Free radicals, lipid peroxidation and sperm function. Reprod. Fertil. Dev. 1995, 7, 659–668.
- Kodama, H.; Kuribayashi, Y.; Gagnon, C. Effect of sperm lipid peroxidation on fertilization. J. Androl. 1996, 17, 151–157.
- Griveau, J.F.; Le Lannou, D. Reactive oxygen species and human spermatozoa: Physiology and pathology. Int. J. Androl. 1997, 20, 61–69.
- Armstrong, J.S.; Rajasekaran, M.; Chamulitrat, W.; Gatti, P.; Hellstrom, W.J.; Sikka, S.C. Characterization of reactive oxygen species induced e_ects on human spermatozoa movement and energy metabolism. Free Radic. Biol. Med. 1999, 26, 869–880.
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10.
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, 163–174.
- Aitken, R.J.; Drevet, J.R. The importance of oxidative stress in determining the functionality of mammalian spermatozoa: A two-edged sword. Antioxidants 2020, 9, 111.
- Weir, C.P.; Robaire, B. Spermatozoa have decreased antioxidant enzymatic capacity and increased reactive oxygen species production during aging in the Brown Norway rat. J. Androl. 2007, 28, 229–240.
- Bourgeron, T. Mitochondrial function and male infertility. Results Probl. Cell Differ. 2000, 28, 187–210.
- Kehrer, J.P.; Robertson, J.D.; Smith, C.V. Free Radicals and Reactive Oxygen Species. Compr. Toxicol. 2010, 1, 277–307.
- Twigg, J.; Fulton, N.; Gomez, E.; Irvine, D.S.; Aitken, R.J. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: Lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum. Reprod. 1998, 13, 1429–1436.
- Sanocka, D.; Kurpisz, M. Reactive oxygen species and sperm cells. Reprod. Biol. Endocrinol. 2004, 2, 12.
- Mennella, M.R.; Jones, R. Properties of spermatozoal superoxide dismutase and lack of involvement of superoxides in metal-ion-catalysed lipid-peroxidation and reactions in semen. Biochem. J. 1980, 191, 289–297.
- Zini, A.; de Lamirande, E.; Gagnon, C. Reactive oxygen species in semen of infertile patients: Levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int. J. Androl. 1993, 16, 183–188.
- Vernet, P.; Aitken, R.J.; Drevet, J.R. Antioxidant strategies in the epididymis. Mol. Cell Endocrinol. 2004, 216, 31–39.





