
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Davide Vione | + 4618 word(s) | 4618 | 2021-05-08 08:12:22 | | | |
| 2 | Vivi Li | Meta information modification | 4618 | 2021-05-28 03:50:11 | | |
Video Upload Options
Aromatic nitroderivatives are compounds of considerable environmental concern, because some of them are phytotoxic (especially the nitrophenols, and particularly 2,4-dinitrophenol), others are mutagenic and potentially carcinogenic (e.g., the nitroderivatives of polycyclic aromatic hydrocarbons, such as 1-nitropyrene), and all of them absorb sunlight as components of the brown carbon. The latter has the potential to affect the climatic feedback of atmospheric aerosols. Most nitroderivatives are secondarily formed in the environment and, among their possible formation processes, photonitration upon irradiation of nitrate or nitrite is an important pathway that has periodically gained considerable attention.
1. Introduction
The photonitration process is the formation of nitroderivatives from suitable substrates under irradiation conditions [1]. Irradiation is usually essential to the formation of the reactive species (nitrating agents), but in some cases it may also play a key role in activating the substrate to the nitration reaction, which would otherwise not take place in the dark with the same nitrating agent (vide infra for the case of the nitration of nitrophenols) [2].
Starting from earlier works in the early 1980s [3][4], studies on photonitration in aqueous solution have focused on processes that are triggered by the photochemistry of nitrate and nitrite ions. The fact that the reaction takes place in the aqueous phase, the wide environmental occurrence of both nitrate and nitrite, as well as the ability of both ions to absorb environmental UV radiation from sunlight [5], explain why the photonitration reactions have attracted interest, in an attempt to account for the formation of nitroderivatives in surface waters and, most notably, in atmospheric waters and aerosols.
In particular, there has been interest towards photonitration in three successive periods—(i) from the early 1980s to the mid-1990s, when the main goal was to account for the formation of phytotoxic nitrophenols that, together with acid rains, play a role in forest decline [6][7]; (ii) the 2000s, when the process attracted attention to account for the occurrence (and secondary formation) of toxic, mutagenic and potentially carcinogenic nitroaromatic compounds in atmospheric aerosols [8][9]; and (iii) the present time, when most interest is accounted for by the fact that the –NO2 group of nitroderivatives enables them to absorb sunlight (visible radiation in addition to the UV, because most nitroderivatives are yellow both as solids and in aqueous solution). As part of the so-called “brown carbon”, nitroaromatic compounds play a role in the absorption of sunlight by airborne aerosols and, by so doing, they affect to a potentially significant extent the still-debated and insufficiently defined climate feedback of the aerosols [10][11][12][13][14][15][16]. Another reason for the recent interest towards photonitration is the acknowledgement of the important role it may play in photochemical processes for water treatment, which are gaining increasing attention and where the occurrence of nitrate and nitrite under technical UV irradiation might favour the formation of harmful compounds [17][18][19][20][21].
Whatever the reason for the focus on the photonitration reactions, most of the relevant studies had/have to address the issue of the photonitration pathway(s). On the one side, this task is apparently made easy by the fact that both nitrate and nitrite are able to produce the nitrating agent •NO2 (nitrogen dioxide) under irradiation [22]. However, the photonitration process has several features (effect of pH, because photonitration is usually enhanced under acidic conditions, and effect of the addition of scavengers of the hydroxyl radical, •OH, which are very common in the natural environment) that often have to be explained as well. Actually, the attempts to account for the whole features of the photonitration process, based on the role of •NO2 as the sole nitrating agent, and on the pH effect as only affecting the production of •NO2 by nitrate and nitrite have proven, on the whole, largely unsatisfactory [1]. To make things worse, photonitration appeared to induce the formation of nitroderivatives from substrates that were too electron-poor to react with •NO2 [2]. Indeed, more detailed studies have shown that irradiation of both nitrate and nitrite produces a plethora of potential nitrating agents [23][24], in different amounts (also depending on the conditions, e.g., the pH of the solution) and with different reactivity, which interact with each substrate to finally produce the observed effects. Therefore, the photonitration process is not amenable to a simple explanation, based on the role of a single nitrating agent.
Unfortunately, essential information about the photonitration pathway(s) has been fragmented in several different publications, and there is currently not a single paper where all the mechanistic findings are summarised and compared. This review paper is intended to fill this gap, also in view of the recent interest towards the photochemical formation of nitroderivatives as brown-carbon components, and as potential contaminants of treated water and wastewater. Therefore, researchers working in the photonitration field could find here a reference and a guide through an environmentally interesting but, at the same time, very tricky process. This review paper deals with the photonitration of aromatic compounds, which has been the main research focus so far. However, it is important to point out that the formation of nitro- and nitrosoderivatives under irradiation has also been observed in the presence of aliphatic precursors such as primary amines [25], which have the potential to yield toxic and carcinogenic compounds.
2. Aromatic Photonitration in Aqueous Solution
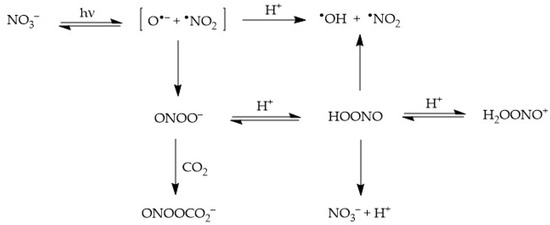
2.1. Nitrate (NO3−) Photolysis
-
Diffusion of •NO2 + O•− out of the solvent cage and into the solution bulk, where O•− undergoes prompt protonation to •OH if pH < 12;
-
Geminate recombination of the two fragments back to the original NO3−; and
-
Recombination of the fragments to form peroxynitrite, ONOO−, which is an isomer of nitrate (this latter process takes the form of nitrate photoisomerisation). Peroxynitrite is the conjugate base of the weak acid HOONO (pKa = 7), peroxynitrous acid. HOONO is an unstable species that can either give back nitrate or decompose into •OH + •NO2. In contrast, OONO− mainly reacts with dissolved CO2 [24].
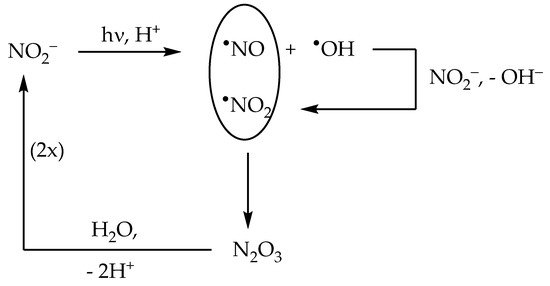
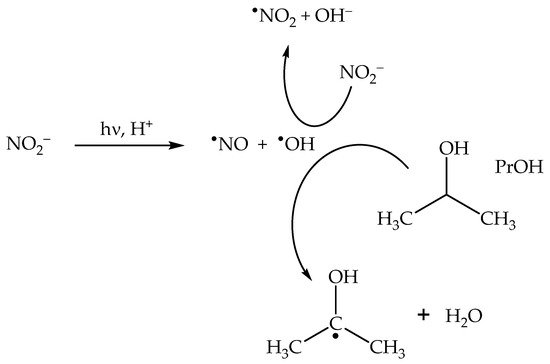
2.2. Nitrite (NO2−) Photolysis
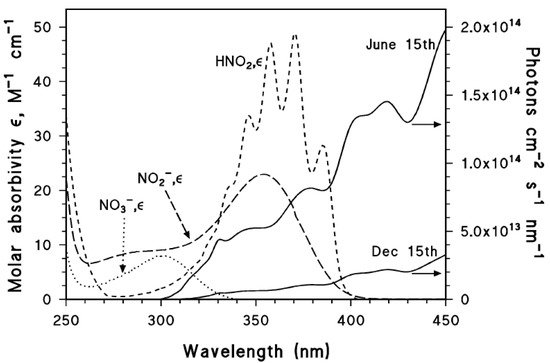
2.3. A Brief Overview of the Different Photogenerated Nitrating Agents
2.4. Phenol Photonitration Pathways
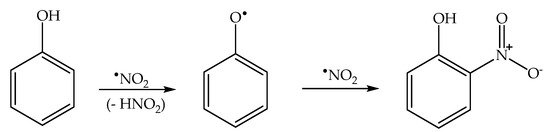
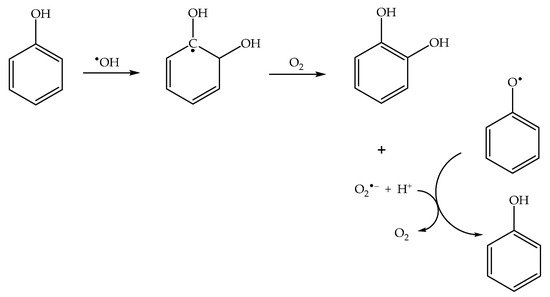
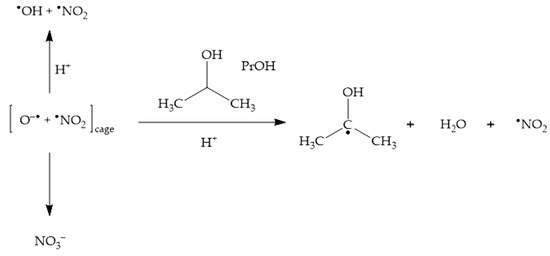
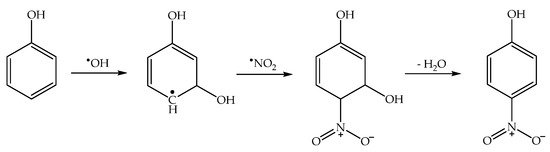
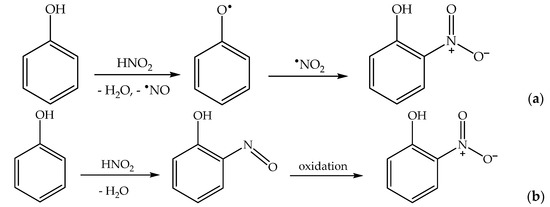
2.5. The Photonitration of Nitrophenols
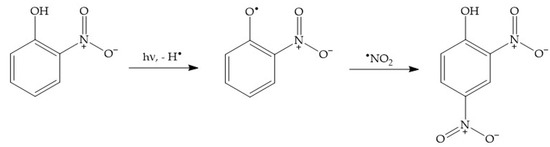
2.6. Photonitration by HOONO/H2OONO+: The Case of Naphthalene/NO3−/hν
2.7. Nitrosation/Oxidation by Photogenerated HNO2: The Cases of Catechol and 1-Naphthol with NO3−/hν
2.8. Proposal of a Protocol for the Study of Photonitration Pathways
-
In neutral solution, there is a real chance for •NO2 to be involved in photonitration (especially with electron-rich substrates). Addition of •OH scavengers such as alcohols may help supporting the possible involvement of •NO2—indeed, photonitration by nitrate should be enhanced upon addition of the scavengers, if it involves •NO2 (solvent-cage effect, see Scheme 1). In contrast, •OH scavengers should inhibit photonitration by nitrite (•OH is needed to oxidise NO2− to •NO2, see Scheme 2);
-
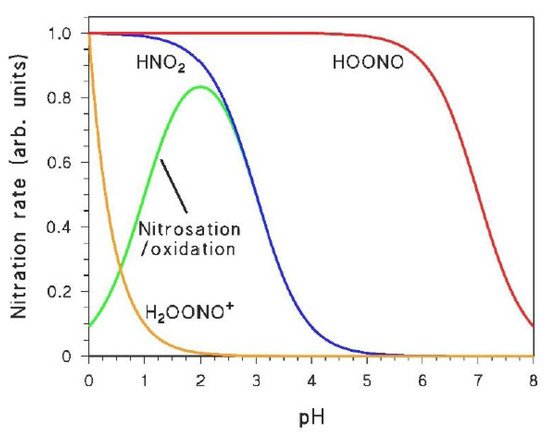
The effect of pH is key to differentiate between different reactive species, especially in the case of nitrate irradiation. Therefore, photonitration rates should be studied as a function of pH with irradiated nitrate, comparing the results with those reported in Figure 2. In several cases, such a comparison will give a clue as to which reactive species is mostly involved in the process;
-
If the pH trend of photonitration by NO3−/UV suggests the possible involvement of HNO2, it is advisable to directly study the reactivity of the substrate with HNO2 in the dark (HNO2 is produced by acidifying an NO2− solution). Direct nitration by HNO2 is studied by taking into account the initial formation rates of the nitroderivatives, while the nitrosation/oxidation process is highlighted by quantifying the concentration values reached by the nitroderivatives after a certain amount of time (e.g., 30 to 60 min reaction time in the dark). Note that the oxidation of the nitrosoderivatives might be faster with NO3− under irradiation than with HNO2 in the dark;
-
If, in contrast, the pH trend of photonitration suggests the involvement of HOONO or H2OONO+, one should study the dark reactivity of these species. HOONO can be produced in the dark by the reaction of H2O2 + HNO2 [50]. An important issue is that the dark formation of HOONO involves H3O2+ + HNO2, thus the pH trend of nitroderivatives formed by HOONO in the dark would show an inflection point at pH ~ 1.5 [50], which is not expected to be seen in the corresponding process taking place under irradiation. In contrast, the involvement of H2OONO+ in dark nitration would still produce nitroderivative formation rates that are ∝ [H+].
2.9. Possible Occurrence of Photonitration in Natural Waters
Evidence of the possible occurrence of photonitration processes in the natural environment has been obtained in the case of natural surface waters, although biological nitration pathways cannot be ruled out. In particular, it appears that the environmental conditions of the Rhône delta lagoons (shallow and sunlit waters, elevated concentrations of nitrate and nitrite) are particularly favourable to the transformation of phenolic compounds, arising from the environmental dissipation of phenoxyacid herbicides, into the corresponding nitroderivatives [61][62][63][64][65][66]:
-
2,4-Dichlorophenoxyacetic acid → 2,4-dichlorophenol → 2,4-dichloro-6-nitrophenol
-
MCPA → 4-chloro-2-methylphenol → 4-chloro-2-methyl-6-nitrophenol
-
Dichlorprop → 4-chlorophenol → 2-nitro-4-chlorophenolwhere MCPA = 4-chloro-2-methylphenoxyacetic acid, and dichlorprop = 2-(2,4-dichlorophenoxy)propanoic acid.
It should be remarked that natural waters are indeed much more complex compared to simplified laboratory systems, thus interaction between different components is highly likely. For instance, nitrate and nitrite occur together in the Rhône-delta lagoon water, and the processes they may trigger differ from the mere sum of the separate photoreactions. In particular, the oxidation of NO2− to •NO2 can be carried out by the •OH radicals produced by both nitrate and nitrite photolysis [67]. Moreover, surface waters contain additional components that can affect the process. For example, iron oxides such as hematite (α-Fe2O3) have been shown to remarkably favour the photonitration both of phenol [68] and of the chlorinated phenols that undergo nitration in the Rhône delta lagoons [61][64]. Further, the oxidation of NO2− to •NO2 by •OH can be inhibited by a number of •OH scavengers that occur in natural waters, such as natural organic matter and inorganic carbon. That said, Scheme 10 shows a summary of the possible reaction pathways that might account for the photonitration of 2,4-dichlorophenol into 2,4-dichloro-6-nitrophenol in lagoon waters. Similar reaction pathways are likely to be operational for the other compounds.
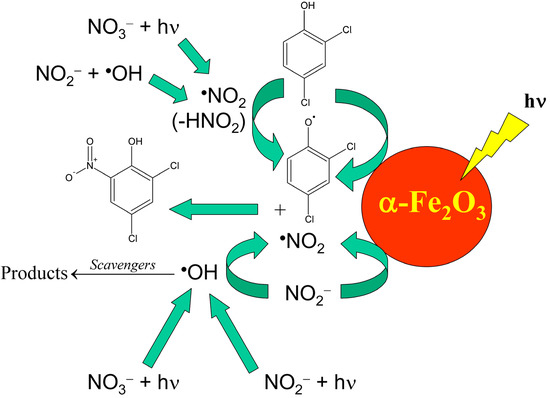
Scheme 10. Environmental reaction pathways that could account for the photochemical nitration of 2,4-dichlorophenol into 2,4-dichloro-6-nitrophenol.
References
- Dzengel, J.; Theurich, J.; Bahnemann, D.W. Formation of nitroaromatic compounds in advanced oxidation processes: Photolysis versus photocatalysis. Environ. Sci. Technol. 1999, 33, 294–300.
- Vione, D.; Maurino, V.; Minero, C.; Pelizzetti, E. Aqueous atmospheric chemistry: Formation of 2,4-dinitrophenol upon nitration of 2-nitrophenol and 4-nitrophenol in solution. Environ. Sci. Technol. 2005, 39, 7921–7931.
- Suzuki, J.; Okazaki, H.; Sato, T.; Suzuki, S. Formation of mutagens by photochemical reaction of biphenyl in nitrate aqueous solution. Chemosphere 1982, 11, 437–444.
- Suzuki, J.; Ueki, T.; Shimizu, S.; Uesugi, K.; Suzuki, S. Formation of mutagens by photolysis of amino acids in neutral aqueous solution containing nitrite or nitrate ion. Chemosphere 1985, 14, 493–500.
- Scholes, R.C.; Prasse, C.; Sedlak, D.L. The role of reactive nitrogen species in sensitized photolysis of wastewater-derived Trace organic contaminants. Environ. Sci. Technol. 2019, 53, 6483–6491.
- Machado, F.; Boule, P. Photonitration and photonitrosation of phenolic derivatives induced in aqueous solution by excitation of nitrite and nitrate ions. J. Photochem. Photobiol. A Chem. 1995, 86, 73–80.
- Natangelo, M.; Mangiapan, S.; Bagnati, R.; Benfenati, E.; Fanelli, R. Increased concentrations of nitrophenols in leaves from a damaged forestal site. Chemosphere 1999, 38, 1495–1503.
- Sugiyama, H.; Watanabe, T.; Hirayama, T. Nitration of pyrene in metallic oxides as soil components in the presence of indoor air, nitrogen dioxide gas, nitrite ion, or nitrate ion under xenon irradiation. J. Health Sci. 2001, 47, 28–35.
- Guidi, G.; Librando, V.; Minniti, Z.; Bolzacchini, E.; Perrini, G.; Bracchitta, G.; Alparone, A.; Catalfo, A. The PAH and nitro-PAH concentration profiles in size-segregated urban particulate matter and soil in traffic-related sites in Catania, Italy. Polycycl. Aromat. Compd. 2012, 32, 439–456.
- Rubio, M.A.; Lissi, E.; Herrera, N.; Pérez, V.; Fuentes, N. Phenol and nitrophenols in the air and dew waters of Santiago de Chile. Chemosphere 2012, 86, 1035–1039.
- Kitanovski, Z.; Čusak, A.; Grgić, I.; Claeys, M. Chemical characterization of the main products formed through aqueous-phase photonitration of guaiacol. Atmos. Meas. Tech. 2014, 7, 2457–2470.
- Kroflič, A.; Grilc, M.; Grgić, I. Unraveling pathways of guaiacol nitration in atmospheric waters: Nitrite, a source of reactive nitronium ion in the atmosphere. Environ. Sci. Technol. 2015, 49, 9150–9158.
- Vidović, K.; Jurković, D.L.; Šala, M.; Kroflič, A.; Grgić, I. Nighttime aqueous-phase formation of nitrocatechols in the atmospheric condensed phase. Environ. Sci. Technol. 2018, 52, 9722–9730.
- Kroflič, A.; Huš, M.; Grilc, M.; Grgić, I. Underappreciated and complex role of nitrous acid in aromatic nitration under mild environmental conditions: The case of activated methoxyphenols. Environ. Sci. Technol. 2018, 52, 13756–13765.
- Vidović, K.; Kroflič, A.; Šala, M.; Grgić, I. Aqueous-phase brown carbon formation from aromatic precursors under sunlight conditions. Atmosphere 2020, 11, 131.
- Pang, H.; Zhang, Q.; Lu, X.; Li, K.; Chen, H.; Chen, J.; Yang, X.; Ma, Y.; Ma, J.; Huang, C. Nitrite-mediated photooxidation of vanillin in atmospheric aqueous phase. Environ. Sci. Technol. 2019, 53, 14253–14263.
- Huang, Y.; Kong, M.; Westerman, D.C.; Xu, E.G.; Coffin, S.; Cochran, K.H.; Liu, Y.; Richardson, S.; Schlenk, D.; Dionysiou, D. Effects of HCO3− on degradation of toxic contaminants of emerging concern by UV/NO3−. Environ. Sci. Technol. 2018, 52, 12697–12707.
- Wu, Z.; Chen, C.; Zhu, B.; Huang, C.; An, T.; Meng, F.; Fang, J. Reactive nitrogen species are also involved in the transformation of micropollutants by the UV/monochloramine process. Environ. Sci. Technol. 2019, 53, 11142–11152.
- Xu, L.; Sun, Y.; Gan, L.; Han, J.; Wang, P.; Yu, L.; Mei, X.; Li, W.; Lyu, B.; Pei, C.; et al. Utilization of photochemical circulation between NO3− and NO2− in water to degrade photoinert dimethyl phthalate: Influence of organic media and mechanism study. Appl. Catal. B Environ. 2019, 259, 117958.
- Zhou, S.; Li, L.; Wu, Y.; Zhu, S.; Zhu, N.; Bu, L.; Dionysiou, D. UV365 induced elimination of contaminants of emerging concern in the presence of residual nitrite: Roles of reactive nitrogen species. Water Res. 2020, 178, 115829.
- Chen, C.; Wu, Z.; Zheng, S.; Wang, L.; Niu, X.; Fang, J. Comparative study for interactions of sulfate radical and hydroxyl radical with phenol in the presence of nitrite. Environ. Sci. Technol. 2020, 54, 8455–8463.
- Mack, J.; Bolton, J. Photochemistry of nitrite and nitrate in aqueous solution: A review. J. Photochem. Photobiol. A Chem. 1999, 128, 1–13.
- Vione, D.; Sur, B.; Dutta, B.K.; Maurino, V.; Minero, C. On the effect of 2-propanol on phenol photonitration upon nitrate photolysis. J. Photochem. Photobiol. A Chem. 2011, 224, 68–70.
- Carena, L.; Vione, D. Photochemical reaction of peroxynitrite and carbon dioxide could account for up to 15% of carbonate radicals generation in surface waters. Environ. Chem. Lett. 2016, 14, 183–187.
- Ohta, T.; Suzuki, J.; Iwano, Y.; Suzuki, S. Photochemical nitrosation of dimethylamine in aqueous solution containing nitrite. Chemosphere 1982, 11, 797–801.
- Chen, L.; Kong, L.; Tong, S.; Yang, K.; Jin, S.; Wang, C.; Wang, L. Aqueous phase oxidation of bisulfite influenced by nitrate photolysis. Atmos. Chem. Phys. 2020, 1–40.
- Bianco, A.; Passananti, M.; Brigante, M.; Mailhot, G. Photochemistry of the cloud aqueous phase: A review. Molecules 2020, 25, 423.
- Mariotti, M.; Rogowska-Wrzesińska, A.; Hägglund, P.; Davies, M.J. Cross-linking and modification of fibronectin by peroxynitrous acid: Mapping and quantification of damage provides a new model for domain interactions. J. Biol. Chem. 2021, 296, 100360.
- Benedict, K.; McFall, A.S.; Anastasio, C. Quantum yield of nitrite from the photolysis of aqueous nitrate above 300 nm. Environ. Sci. Technol. 2017, 51, 4387–4395.
- Yang, W.; Han, C.; Yang, H.; Xue, X. Significant HONO formation by the photolysis of nitrates in the presence of humic acids. Environ. Pollut. 2018, 243, 679–686.
- Bedini, A.; Maurino, V.; Minero, C.; Vione, D. Theoretical and experimental evidence of the photonitration pathway of phenol and 4-chlorophenol: A mechanistic study of environmental significance. Photochem. Photobiol. Sci. 2012, 11, 418–424.
- Maiti, B.K.; Maia, L.; Moura, I.; Moura, J. NiII -ATCUN-catalyzed tyrosine nitration in the presence of nitrite and sulfite. Chem. Eur. J. 2019, 25, 4309–4314.
- De Laurentiis, E.; Minella, M.; Berto, S.; Maurino, V.; Minero, C.; Vione, D. The fate of nitrogen upon nitrite irradiation: Formation of dissolved vs. gas-phase species. J. Photochem. Photobiol. A Chem. 2015, 307, 30–34.
- Fu, X.; Wang, T.; Zhang, L.; Li, Q.; Wang, Z.; Xia, M.; Yun, H.; Wang, W.; Yu, C.; Yue, D.; et al. The significant contribution of HONO to secondary pollutants during a severe winter pollution event in southern China. Atmos. Chem. Phys. 2019, 19, 1–14.
- Vione, D.; Minero, C.; Housari, F.; Chirón, S. Photoinduced transformation processes of 2,4-dichlorophenol and 2,6-dichlorophenol on nitrate irradiation. Chemosphere 2007, 69, 1548–1554.
- Vione, D.; Maurino, V.; Minero, C.; Pelizzetti, E. Nitration and photonitration of naphthalene in aqueous systems. Environ. Sci. Technol. 2005, 39, 1101–1110.
- Degendorfer, G.; Chuang, C.; Mariotti, M.; Hammer, A.; Hoefler, G.; Hägglund, P.; Malle, E.; Wise, S.G.; Davies, M. Exposure of tropoelastin to peroxynitrous acid gives high yields of nitrated tyrosine residues, di-tyrosine cross-links and altered protein structure and function. Free Rad. Biol. Med. 2018, 115, 219–231.
- Campolo, N.; Issoglio, F.; Estrin, D.; Bartesaghi, S.; Radi, R. 3-Nitrotyrosine and related derivatives in proteins: Precursors, radical intermediates and impact in function. Essays Biochem. 2020, 64, 111–133.
- Minero, C.; Bono, F.; Rubertelli, F.; Pavino, D.; Maurino, V.; Pelizzetti, E.; Vione, D. On the effect of pH in aromatic photonitration upon nitrate photolysis. Chemosphere 2007, 66, 650–656.
- Vione, D.; De Laurentiis, E.; Berto, S.; Minero, C.; Hatipoglu, A.; Çınar, Z. Modeling the photochemical transformation of nitrobenzene under conditions relevant to sunlit surface waters: Reaction pathways and formation of intermediates. Chemosphere 2016, 145, 277–283.
- Mekic, M.; Liu, J.; Zhou, W.; Loisel, G.; Cai, J.; He, T.; Jiang, B.; Yu, Z.; Lazarou, Y.; Li, X.; et al. Formation of highly oxygenated multifunctional compounds from cross-reactions of carbonyl compounds in the atmospheric aqueous phase. Atmos. Environ. 2019, 219, 117046.
- Hayyan, M.; Hashim, M.A.; Al Nashef, I. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085.
- Wang, F. Reactivity of the Solvated Electron, the Hydroxyl Radical and Its Precursor in Deuterated Water Studied by Picosecond Pulse Radiolysis. Ph.D. Thesis, Université Paris Saclay, Paris, France, 2018; p. 179.
- Nayebzadeh, M.; Vahedpour, M. A Review on reactions of polycyclic aromatic hydrocarbons with the most abundant atmospheric chemical fragments: Theoretical and experimental data. Prog. React. Kinet. Mech. 2017, 42, 201–220.
- Mao, X.; Wang, S.; Huang, Y.; Zhou, T. A theoretical investigation of gas phase OH-initiated acenaphthylene degradation reaction. Comput. Chem. 2017, 5, 22–37.
- Rubio, M.A.; Lissi, E.; Olivera, N.; Reyes, J.; López-Alarcón, C. Reactions of p-substituted phenols with nitrous acid in aqueous solution. Int. J. Chem. Kinet. 2014, 46, 143–150.
- Takahama, U.; Hirota, S. Possible reactions of dietary phenolic compounds with salivary nitrite and thiocyanate in the stomach. Antioxidants 2017, 6, 53.
- Vione, D.; Maurino, V.; Minero, C.; Pelizzetti, E. Phenol photonitration. Ann. Chim. 2002, 92, 919–929.
- Vione, D.; Maurino, V.; Minero, C.; Pelizzetti, E. Phenol photonitration upon UV irradiation of nitrite in aqueous solution II: Effects of pH and TiO2. Chemosphere 2001, 45, 903–910.
- Vione, D.; Maurino, V.; Minero, C.; Borghesi, D.; Lucchiari, M.; Pelizzetti, E. New processes in the environmental chemistry of nitrite. 2. The role of hydrogen peroxide. Environ. Sci. Technol. 2003, 37, 4635–4641.
- Bartesaghi, S.; Radi, R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol. 2018, 14, 618–625.
- Harrison, M.; Barra, S.; Borghesi, D.; Vione, D.; Arsene, C.; Olariu, R. Nitrated phenols in the atmosphere: A review. Atmos. Environ. 2005, 39, 231–248.
- Daviere, A.; Sotomski, M.; Audibert, A.; Carol, P.; Hubert, S.; Lebreton, S.; Louvet-Vallée, S.; Pédron, J.; Puyaubert, J.; Kraepiel, Y.; et al. Synergistic toxicity between glyphosate and 2,4-dinitrophenol on budding yeast is not due to H2O2-mediated oxidative stress. Matters 2019.
- Barsotti, F.; Bartels-Rausch, T.; De Laurentiis, E.; Ammann, M.; Brigante, M.; Mailhot, G.; Maurino, V.; Minero, C.; Vione, D. Photochemical formation of nitrite and nitrous acid (HONO) upon irradiation of nitrophenols in aqueous solution and in viscous secondary organic aerosol proxy. Environ. Sci. Technol. 2017, 51, 7486–7495.
- Vione, D.; Maurino, V.; Minero, C.; Lucchiari, M.; Pelizzetti, E. Nitration and hydroxylation of benzene in the presence of nitrite/nitrous acid in aqueous solution. Chemosphere 2004, 56, 1049–1059.
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental implications of hydroxyl radicals (•OH). Chem. Rev. 2015, 115, 13051–13092.
- Duan, L.; Zhang, T.; Song, W.; Jiang, C.; Hou, Y.; Zhao, W.; Chen, W.; Alvarez, P. Photolysis of graphene oxide in the presence of nitrate: Implications for graphene oxide integrity in water and wastewater treatment. Environ. Sci. Nano 2019, 6, 136–145.
- Vione, D.; Maurino, V.; Pelizzetti, E.; Minero, C. Phenol photonitration and photonitrosation upon nitrite photolysis in basic solution. Int. J. Environ. Anal. Chem. 2004, 84, 493–504.
- Lorentzen, L.; Chuang, C.; Rogowska-Wrzesińska, A.; Davies, M. Identification and quantification of sites of nitration and oxidation in the key matrix protein laminin and the structural consequences of these modifications. Redox Biol. 2019, 24, 101226.
- Taraborrelli, D.; Cabrera-Perez, D.; Bacer, S.; Gromov, S.; Lelieveld, J.; Sander, R.; Pozzer, A. Influence of aromatics on tropospheric gas-phase composition. Atmos. Chem. Phys. 2020, 2615–2636.
- Chirón, S.; Minero, C.; Vione, D. Occurrence of 2,4-dichlorophenol and of 2,4-dichloro-6-nitrophenol in the Rhône River Delta (Southern France). Environ. Sci. Technol. 2007, 41, 3127–3133.
- Xia, Z.; Zhang, L.; Zhao, Y.; Yan, X.; Li, S.; Gu, T.; Jiang, J. Biodegradation of the herbicide 2,4-dichlorophenoxyacetic acid by a new isolated strain of Achromobacter sp. LZ35. Curr. Microbiol. 2016, 74, 193–202.
- Nowak, K.; Miltner, A.; Poll, C.; Kandeler, E.; Streck, T.; Pagel, H. Plant litter enhances degradation of the herbicide MCPA and increases formation of biogenic non-extractable residues in soil. Environ. Int. 2020, 142, 105867.
- Chirón, S.; Comoretto, L.; Rinaldi, E.; Maurino, V.; Minero, C.; Vione, D. Pesticide by-products in the Rhône delta (Southern France). The case of 4-chloro-2-methylphenol and of its nitroderivative. Chemosphere 2009, 74, 599–604.
- Muszyński, P.; Brodowska, M.; Paszko, T. Occurrence and transformation of phenoxy acids in aquatic environment and photochemical methods of their removal: A review. Environ. Sci. Pollut. Res. 2019, 27, 1276–1293.
- Maddigapu, P.R.; Vione, D.; Ravizzoli, B.; Minero, C.; Maurino, V.; Comoretto, L.; Chirón, S. Laboratory and field evidence of the photonitration of 4-chlorophenol to 2-nitro-4-chlorophenol and of the associated bicarbonate effect. Environ. Sci. Pollut. Res. 2010, 17, 1063–1069.
- Xu, H.; Li, Y.; Liu, J.; Du, H.; Du, Y.; Su, Y.; Jiang, H. Photogeneration and steady-state concentration of hydroxyl radical in river and lake waters along middle-lower Yangtze region, China. Water Res. 2020, 176, 115774.
- Vione, D.; Maurino, V.; Minero, C.; Pelizzetti, E. New processes in the environmental chemistry of nitrite: Nitration of phenol upon nitrite photoinduced oxidation. Environ. Sci. Technol. 2002, 36, 669–676.




