Aromatic nitroderivatives are compounds of considerable environmental concern, because some of them are phytotoxic (especially the nitrophenols, and particularly 2,4-dinitrophenol), others are mutagenic and potentially carcinogenic (e.g., the nitroderivatives of polycyclic aromatic hydrocarbons, such as 1-nitropyrene), and all of them absorb sunlight as components of the brown carbon. The latter has the potential to affect the climatic feedback of atmospheric aerosols. Most nitroderivatives are secondarily formed in the environment and, among their possible formation processes, photonitration upon irradiation of nitrate or nitrite is an important pathway that has periodically gained considerable attention.
- nitroaromatic compounds
- nitrophenols
- atmospheric chemistry
- environmental photochemistry
- secondary contaminants
- photogenerated nitrating agents
1. Introduction
The photonitration process is the formation of nitroderivatives from suitable substrates under irradiation conditions [1]. Irradiation is usually essential to the formation of the reactive species (nitrating agents), but in some cases it may also play a key role in activating the substrate to the nitration reaction, which would otherwise not take place in the dark with the same nitrating agent (vide infra for the case of the nitration of nitrophenols) [2].
Starting from earlier works in the early 1980s [3][4][3,4], studies on photonitration in aqueous solution have focused on processes that are triggered by the photochemistry of nitrate and nitrite ions. The fact that the reaction takes place in the aqueous phase, the wide environmental occurrence of both nitrate and nitrite, as well as the ability of both ions to absorb environmental UV radiation from sunlight [5], explain why the photonitration reactions have attracted interest, in an attempt to account for the formation of nitroderivatives in surface waters and, most notably, in atmospheric waters and aerosols.
In particular, there has been interest towards photonitration in three successive periods—(i) from the early 1980s to the mid-1990s, when the main goal was to account for the formation of phytotoxic nitrophenols that, together with acid rains, play a role in forest decline [6][7][6,7]; (ii) the 2000s, when the process attracted attention to account for the occurrence (and secondary formation) of toxic, mutagenic and potentially carcinogenic nitroaromatic compounds in atmospheric aerosols [8][9][8,9]; and (iii) the present time, when most interest is accounted for by the fact that the –NO2 group of nitroderivatives enables them to absorb sunlight (visible radiation in addition to the UV, because most nitroderivatives are yellow both as solids and in aqueous solution). As part of the so-called “brown carbon”, nitroaromatic compounds play a role in the absorption of sunlight by airborne aerosols and, by so doing, they affect to a potentially significant extent the still-debated and insufficiently defined climate feedback of the aerosols [10][11][12][13][14][15][16][10,11,12,13,14,15,16]. Another reason for the recent interest towards photonitration is the acknowledgement of the important role it may play in photochemical processes for water treatment, which are gaining increasing attention and where the occurrence of nitrate and nitrite under technical UV irradiation might favour the formation of harmful compounds [17][18][19][20][21][17,18,19,20,21].
Whatever the reason for the focus on the photonitration reactions, most of the relevant studies had/have to address the issue of the photonitration pathway(s). On the one side, this task is apparently made easy by the fact that both nitrate and nitrite are able to produce the nitrating agent •NO2 (nitrogen dioxide) under irradiation [22]. However, the photonitration process has several features (effect of pH, because photonitration is usually enhanced under acidic conditions, and effect of the addition of scavengers of the hydroxyl radical, •OH, which are very common in the natural environment) that often have to be explained as well. Actually, the attempts to account for the whole features of the photonitration process, based on the role of •NO2 as the sole nitrating agent, and on the pH effect as only affecting the production of •NO2 by nitrate and nitrite have proven, on the whole, largely unsatisfactory [1]. To make things worse, photonitration appeared to induce the formation of nitroderivatives from substrates that were too electron-poor to react with •NO2 [2]. Indeed, more detailed studies have shown that irradiation of both nitrate and nitrite produces a plethora of potential nitrating agents [23][24][23,24], in different amounts (also depending on the conditions, e.g., the pH of the solution) and with different reactivity, which interact with each substrate to finally produce the observed effects. Therefore, the photonitration process is not amenable to a simple explanation, based on the role of a single nitrating agent.
Unfortunately, essential information about the photonitration pathway(s) has been fragmented in several different publications, and there is currently not a single paper where all the mechanistic findings are summarised and compared. This review paper is intended to fill this gap, also in view of the recent interest towards the photochemical formation of nitroderivatives as brown-carbon components, and as potential contaminants of treated water and wastewater. Therefore, researchers working in the photonitration field could find here a reference and a guide through an environmentally interesting but, at the same time, very tricky process. This review paper deals with the photonitration of aromatic compounds, which has been the main research focus so far. However, it is important to point out that the formation of nitro- and nitrosoderivatives under irradiation has also been observed in the presence of aliphatic precursors such as primary amines [25], which have the potential to yield toxic and carcinogenic compounds.
2. Aromatic Photonitration in Aqueous Solution
2.1. Nitrate (NO3−) Photolysis
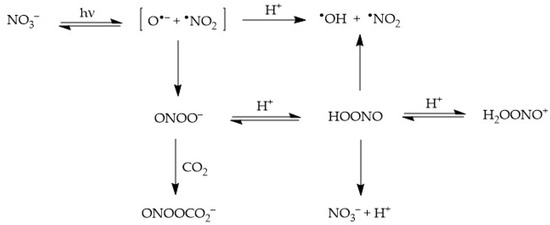
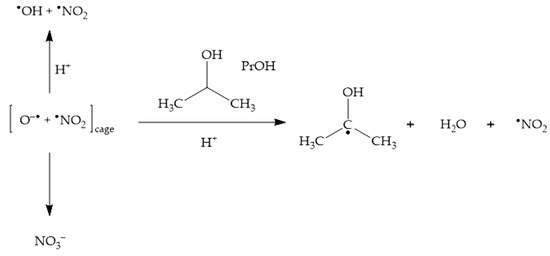
Nitrate absorbs sunlight up to approximately 340 nm. It has a weak absorption maximum at 302 nm (εmax = 7.2 L mol−1 cm−1) and, because the irradiance of sunlight increases with increasing wavelength in the UV region, one gets that sunlight absorption by nitrate is most effective at around 315–320 nm. Radiation absorption breaks the NO3− ion into two fragments, namely O•− and •NO2, where O•− is the conjugate base of the hydroxyl radical, •OH [22][26][22,32]:-
Diffusion of •NO2 + O•− out of the solvent cage and into the solution bulk, where O•− undergoes prompt protonation to •OH if pH < 12;
-
Geminate recombination of the two fragments back to the original NO3−; and
-
Recombination of the fragments to form peroxynitrite, ONOO−, which is an isomer of nitrate (this latter process takes the form of nitrate photoisomerisation). Peroxynitrite is the conjugate base of the weak acid HOONO (pKa = 7), peroxynitrous acid. HOONO is an unstable species that can either give back nitrate or decompose into •OH + •NO2. In contrast, OONO− mainly reacts with dissolved CO2 [24].
2.2. Nitrite (NO2−) Photolysis
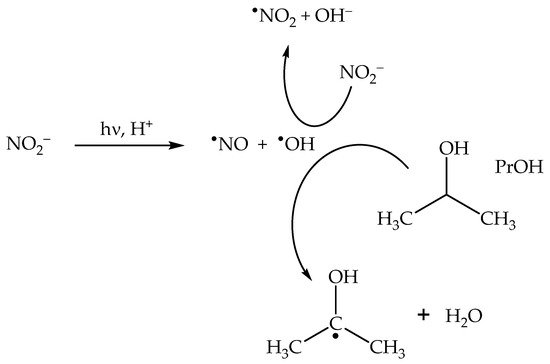
The nitrite anion has two main absorption bands in the environmental UV region. The lower-wavelength band appears as a 270–280 nm shoulder in the nitrite absorption spectrum, and it extends into the UVB region where it overlaps with the higher-wavelength band. The latter produces a clear absorption maximum at around 365 nm. Radiation absorption by nitrite in the environmental UV region (both UVB and UVA) causes photolysis with generation of •NO + O•− as the primary process, and of •NO2 + e−aq as a secondary one [22]: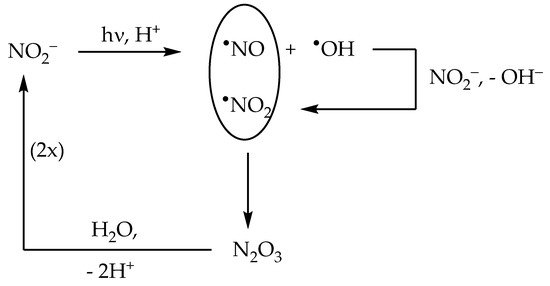
2.3. A Brief Overview of the Different Photogenerated Nitrating Agents
As shown in the previous sections, the irradiation of nitrate and nitrite yields different nitrating agents such as •NO2, HNO2, and HOONO (which can be partially protonated to H2OONO+ in strongly acidic conditions). Electron-rich aromatics will mostly be nitrated by •NO2 or HNO2 that are formed or occur in a higher amount [6][35][6,26], while HOONO/H2OONO+ (however powerful their potential towards aromatic nitration may be [36][37][38][40,41,42]) are produced with low quantum yield by nitrate irradiation. Therefore, the reactivity of HOONO/H2OONO+ will only be highlighted in the presence of deactivated aromatic compounds that do not react with •NO2/HNO2 [2]. The role of the different nitrating agents in the photonitration of a given compound can be highlighted when taking into account the peculiar pH trends that characterise at least some of them. Indeed, H2OONO+ has pKa < 0, HOONO has pKa ~ 7, while HNO2 has pKa ~ 3.5 [22][36][22,40]. Moreover, HNO2 can also be involved in a nitrosation/oxidation process that gives a maximum in nitroaromatic formation at pH 2–3 [39][43]. Figure 2 shows the pH trends that characterise the occurrence and/or reactivity of the different nitrating agents, and that may be reflected in the rates of nitroaromatic formation as a function of pH. Indeed, a study of the pH trend should be a key step when trying to understand the aromatic photonitration pathways (vide infra).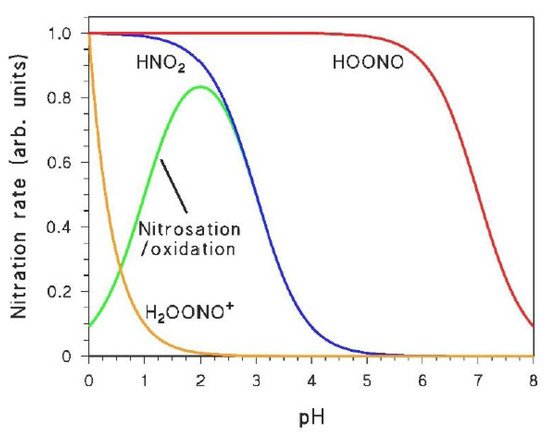
2.4. Phenol Photonitration Pathways
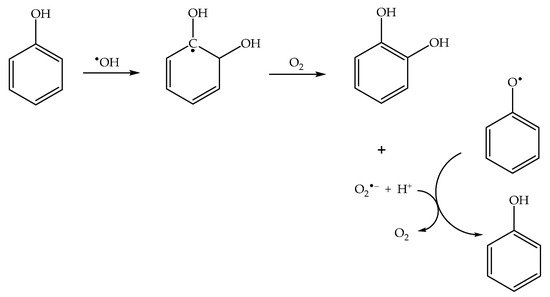
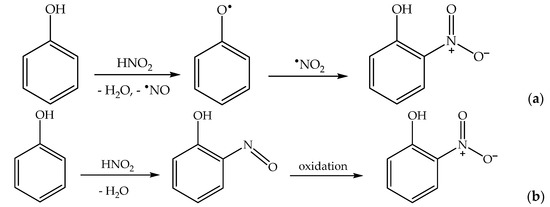
Formation of the ortho and para nitrophenol isomers (2-nitrophenol and 4-nitrophenol, hereinafter respectively 2NP and 4NP) from phenol has been observed upon irradiation of both nitrate and nitrite [1][6][1,6]. In neutral solution, in both cases, the photonitration process has been shown to involve a reaction between phenol and •NO2, with the likely involvement of the phenoxyl radical as a reaction intermediate (Scheme 3). This most likely reaction pathway has been suggested by quantum chemistry calculations as well [31][27].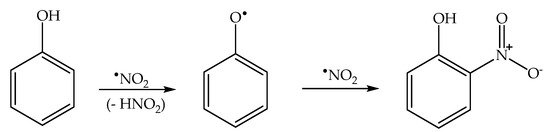
2.5. The Photonitration of Nitrophenols
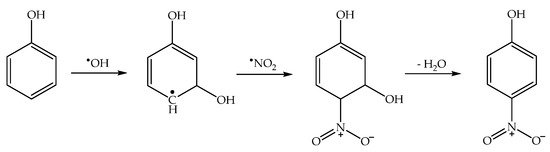
Phenol is amenable to photonitration as shown in the previous section, but this activated aromatic compound can be nitrated under a very wide variety of conditions, both thermal (which in atmospheric waters might also involve N2O5, produced by reaction of •NO3 + •NO2, in addition to HNO2), and photochemical [52][56]. Therefore, secondary environmental nitrophenols might in principle arise from a plethora of nitration pathways, of which photonitration is only one of the possibilities. In contrast, it is quite difficult to account for the nitration of nitrophenols (2NP, 4NP) to 2,4-dinitrophenol (24DNP), i.e., the most phytotoxic nitrophenol compound [52][53][56,57], without invoking the photoinduced nitration pathways. For instance, dark nitration of nitrophenols by •NO2 or HNO2 can be excluded, because neither reactant is able to oxidise either 2NP or 4NP to the corresponding nitrophenoxyl radical [2]. In contrast, photonitration is enabled by the fact that nitrophenols absorb UV-Vis radiation, which induces their photolysis to form the corresponding nitrophenoxyl radicals as the primary reaction intermediates [54][58]. These radicals can then react with photogenerated •NO2 to finally produce 24DNP (Scheme 9).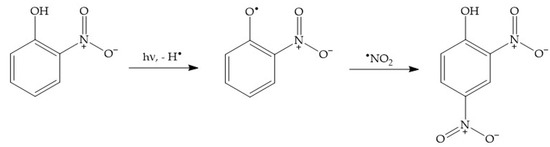
2.6. Photonitration by HOONO/H2OONO+: The Case of Naphthalene/NO3−/hν
Naphthalene is a relatively electron-poor aromatic compound that, similarly to nitrophenols, cannot undergo nitration by either •NO2 or HNO2. However, unlike nitrophenols, naphthalene does not absorb environmental UV radiation, thus, a photolysis/nitration pathway similar to that reported in Scheme 9 cannot be operational in this case. By completely excluding the main nitrating agents produced by nitrate photolysis (•NO2, HNO2), naphthalene could be a good substrate for photonitration induced by the nitrating species produced in a lesser amount (H2OONO+ and HOONO). The available experimental evidence suggests that naphthalene can actually undergo nitration by H2OONO+ to mainly yield 1-nitronaphthalene, and by HOONO to mainly produce 2-nitronaphthalene [36][40]. Because the two nitronaphthalene isomers are produced to a large extent by different nitrating species, one could expect the pH trends of the respective formation rates to be different. Indeed, as suggested by Figure 2, the formation rate of 1-nitronaphthalene was directly proportional to [H+], while that of 2-nitronaphthalene was dependent on pH to a much lesser extent in the tested acidic range [36][40]. For naphthalene, the involvement of H2OONO+ and HOONO in photonitration could be highlighted because of the lack of reactivity with HNO2 and •NO2. Photonitration by HOONO in the presence of NO3−/hν has also been reported for benzene, which is another relatively electron-poor substrate that does not react with either •NO2 or HNO2. The formation rate of nitrobenzene did not show a significant pH trend in the acidic region, as expected for a process involving HOONO (see Figure 2) [55][59].2.7. Nitrosation/Oxidation by Photogenerated HNO2: The Cases of Catechol and 1-Naphthol with NO3−/hν
Nitration of both catechol and 1-naphthol (1-hydroxynaphthalene) was observed upon irradiation in the presence of nitrate, with respective production of 4-nitrocatechol and of 2-nitro-1-naphthol [39][43]. The formation rates of both nitroaromatics under irradiation conditions showed a maximum around pH 3. Both catechol and 1-naphthol react with HNO2 in the dark to yield the mentioned nitroderivatives, but the pH trend of the two nitroaromatic compounds is quite peculiar. In both cases one observes initial formation rates with an inflection point around pH 3.5, as expected by a direct involvement of HNO2 in the nitration process (Figure 2). However, when focusing on the concentration values of the nitroderivatives reached after 30–40 min reaction time, one observes a pH 3 maximum that is very similar to the pH trend observed in the photonitration process. The maximum around pH 3, together with the fact that this phenomenon is observed only after a certain time lapse, suggests that HNO2 triggers a nitrosation/oxidation process in addition to the direct nitration (Figure 2). Coherently, nitrosoderivatives of both catechol and 1-naphthol have been detected in the presence of HNO2 in the dark [39][43]. Overall, the mentioned photonitration process observed in the presence of irradiated nitrate with catechol and 1-naphthol can be accounted for in the hypothesis that the nitrating agent is HNO2, operating mainly through a nitrosation/oxidation pathway [39][43]. Oxidation of the nitrosoderivatives is likely to be faster in the presence of nitrate under irradiation compared to HNO2 in the dark, considering that in the former conditions one also has the photogeneration of •OH, which is a very strong oxidising agent [56][57][60,61]. A nitrosation/oxidation process is also believed to be responsible for the considerable formation of 4-nitrophenol upon irradiation of phenol and nitrite in basic solution (optimal pH for this process is in the range 9–10, and nitrosation of phenol to 4-nitrosophenol is likely induced by •NO2 + •NO instead of HNO2) [58][62].2.8. Proposal of a Protocol for the Study of Photonitration Pathways
As mentioned in previous sections, photonitration is actually a very complex process that stems from the generation of a wide range of different nitrating agents, which differ in the produced amount and intrinsic reactivity. Therefore, the actual production of the nitroderivatives results from the combination of substrate activation to the nitration process, and from the availability of the nitrating agent. However, as a rule of thumb, the electron-rich compounds are more likely to undergo nitration mostly by •NO2 and HNO2 (HOONO and H2OONO+ are also reactive [50][59][54,63], but they occur in a lesser amount). In contrast, electron-poor substrates that are unreactive towards •NO2/HNO2 can only be nitrated by HOONO/H2OONO+. A partial exception to this rule is represented by electron-poor phenols absorbing UV-Vis radiation, such as the nitrophenols. These compounds are unreactive towards •NO2, but radiation absorption yields the corresponding phenoxyl radicals [60][64] that easily produce nitrophenols when reacting with •NO2 itself. In the case of a compound for which reactivity is still unknown, it might be very useful to proceed to the study of the photonitration pathway in a systematic way. Based on previous knowledge, which has been gathered with a range of compounds showing different reactivity towards photonitration, the following actions are foreseen:-
In neutral solution, there is a real chance for •NO2 to be involved in photonitration (especially with electron-rich substrates). Addition of •OH scavengers such as alcohols may help supporting the possible involvement of •NO2—indeed, photonitration by nitrate should be enhanced upon addition of the scavengers, if it involves •NO2 (solvent-cage effect, see Scheme 1). In contrast, •OH scavengers should inhibit photonitration by nitrite (•OH is needed to oxidise NO2− to •NO2, see Scheme 2);
-
The effect of pH is key to differentiate between different reactive species, especially in the case of nitrate irradiation. Therefore, photonitration rates should be studied as a function of pH with irradiated nitrate, comparing the results with those reported in Figure 2. In several cases, such a comparison will give a clue as to which reactive species is mostly involved in the process;
-
If the pH trend of photonitration by NO3−/UV suggests the possible involvement of HNO2, it is advisable to directly study the reactivity of the substrate with HNO2 in the dark (HNO2 is produced by acidifying an NO2− solution). Direct nitration by HNO2 is studied by taking into account the initial formation rates of the nitroderivatives, while the nitrosation/oxidation process is highlighted by quantifying the concentration values reached by the nitroderivatives after a certain amount of time (e.g., 30 to 60 min reaction time in the dark). Note that the oxidation of the nitrosoderivatives might be faster with NO3− under irradiation than with HNO2 in the dark;
-
If, in contrast, the pH trend of photonitration suggests the involvement of HOONO or H2OONO+, one should study the dark reactivity of these species. HOONO can be produced in the dark by the reaction of H2O2 + HNO2 [50][54]. An important issue is that the dark formation of HOONO involves H3O2+ + HNO2, thus the pH trend of nitroderivatives formed by HOONO in the dark would show an inflection point at pH ~ 1.5 [50][54], which is not expected to be seen in the corresponding process taking place under irradiation. In contrast, the involvement of H2OONO+ in dark nitration would still produce nitroderivative formation rates that are ∝ [H+].
2.9. Possible Occurrence of Photonitration in Natural Waters
Evidence of the possible occurrence of photonitration processes in the natural environment has been obtained in the case of natural surface waters, although biological nitration pathways cannot be ruled out. In particular, it appears that the environmental conditions of the Rhône delta lagoons (shallow and sunlit waters, elevated concentrations of nitrate and nitrite) are particularly favourable to the transformation of phenolic compounds, arising from the environmental dissipation of phenoxyacid herbicides, into the corresponding nitroderivatives [61][62][63][64][65][66][31,65,66,67,68,69]:
-
2,4-Dichlorophenoxyacetic acid → 2,4-dichlorophenol → 2,4-dichloro-6-nitrophenol
-
MCPA → 4-chloro-2-methylphenol → 4-chloro-2-methyl-6-nitrophenol
-
Dichlorprop → 4-chlorophenol → 2-nitro-4-chlorophenolwhere MCPA = 4-chloro-2-methylphenoxyacetic acid, and dichlorprop = 2-(2,4-dichlorophenoxy)propanoic acid.
It should be remarked that natural waters are indeed much more complex compared to simplified laboratory systems, thus interaction between different components is highly likely. For instance, nitrate and nitrite occur together in the Rhône-delta lagoon water, and the processes they may trigger differ from the mere sum of the separate photoreactions. In particular, the oxidation of NO2− to •NO2 can be carried out by the •OH radicals produced by both nitrate and nitrite photolysis [67][70]. Moreover, surface waters contain additional components that can affect the process. For example, iron oxides such as hematite (α-Fe2O3) have been shown to remarkably favour the photonitration both of phenol [68][71] and of the chlorinated phenols that undergo nitration in the Rhône delta lagoons [61][64][31,67]. Further, the oxidation of NO2− to •NO2 by •OH can be inhibited by a number of •OH scavengers that occur in natural waters, such as natural organic matter and inorganic carbon. That said, Scheme 10 shows a summary of the possible reaction pathways that might account for the photonitration of 2,4-dichlorophenol into 2,4-dichloro-6-nitrophenol in lagoon waters. Similar reaction pathways are likely to be operational for the other compounds.
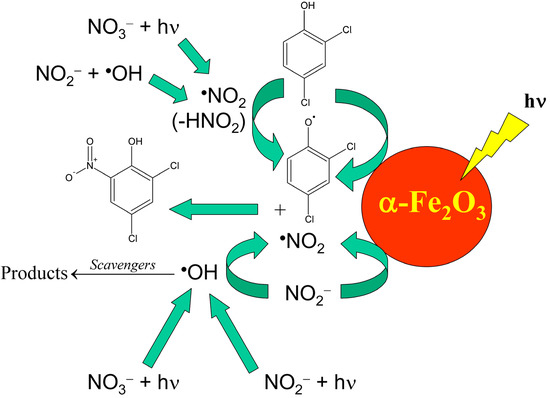
Scheme 10. Environmental reaction pathways that could account for the photochemical nitration of 2,4-dichlorophenol into 2,4-dichloro-6-nitrophenol.
