
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Robert Czarnomysy | + 1764 word(s) | 1764 | 2021-05-26 06:05:48 | | | |
| 2 | Enzi Gong | + 56 word(s) | 1820 | 2021-05-31 04:51:58 | | | | |
| 3 | Enzi Gong | + 62 word(s) | 1882 | 2021-06-21 08:15:57 | | |
Video Upload Options
Se is taken with the diet in the form of organic (SeMet and Sec) and inorganic compounds (selenites, selenides). The absorption of these compounds mostly occurs in the duodenum and cecum, and their absorption mechanism depends on the chemical form of ingested Se. Inorganic compounds are absorbed by simple diffusion (selenites) or by secondary active transport, the so-called cotransport (selenides). In turn, organic compounds (SeMet, Sec, methylselenocysteine (MSC)) are absorbed by active transport in the same way as amino acids.
1. Selenium in the Diet
Selenium (Se) occurs as a trace element in the human body, performing a number of important biological functions, and is essential for life and health [1]. The main natural source of Se is food, but Se content varies. This is determined by the geographical location, soil quality in terms of Se concentration, and how much it is accumulated in plants. The climate and the way the food is cultivated and bred, and how we prepare it for consumption, are also important factors [2][3]. The richest sources of Se in the diet are Se-yeast (Se-enriched yeast), nuts, cereals, organ meats, fish, and seafood [4]. Brazil nuts (Bertholletia excelsa, family Lecythidaceae), a plant from the Amazon, have the highest known Se content among non-Se-enriched food [5]. Table 1 gives the Se content in some examples of food products in our diet.
Table 1. Examples of some food products and their selenium content
| Food | Typical Se Content (µg/g Fresh Weight) | References |
|---|---|---|
| Se-yeast | 3000 | [6] |
| Broccoli (Se-enriched) | 62.3 | [7] |
| Brazil nuts | 0.85–6.86 | [8] |
| Shellfish | 0.36–1.33 | [9] |
| Chicken | 0.57 | [10] |
| Beef | 0.35–0.47 | [11] |
| Lamb | 0.4 | [12] |
| Salmon | 0.21–0.27 | [13] |
| Eggs | 0.17 | [14] |
| Milk products | 0.1–0.55 | [8] |
This microelement can exist in food in both chemical forms—as inorganic and organic compounds [15]. Mostly, Se is found in the form of organic compounds as selenomethionine (SeMet) and selenocysteine (Sec) in plant [16] and animal [17][18] tissues. However, the form of Se intake can affect its bioavailability in the body. Studies show that organic Se (e.g., SeMet) is more bioavailable than its inorganic compounds such as selenates or selenites [18][19][20]. Interestingly, the bioavailability of this element in the body is also modified by race. Richie et al. observed that in whites it is significantly higher than in blacks [21]. Nevertheless, the most important thing is a healthy and balanced diet that contains all the nutrients at the right levels. As far as Se and its recommended daily dose are concerned, it depends on the local content of this element in the cropland, as this affects its content in the food [22][23][24]. According to World Health Organization (WHO) standards, the recommended daily dose of Se for adults is 55 µg/day [4][25][26], while the maximum tolerable adult intake without side effects is set at 400 µg/day [4]. Of course, the requirement for Se depends on age and gender [4], as shown in Table 2.
| Age Group | Male (µg/d) | Female (µg/d) | Pregnancy (µg/d) | Lactation (µg/d) |
|---|---|---|---|---|
| Birth to 6 mths | 15 * | 15 * | ||
| 7 to 12 mths | 20 * | 20 * | ||
| 9 to 13 yrs | 40 | 40 | ||
| 14 to 18 yrs | 55 | 55 | 60 | 70 |
| 19 to 30 yrs | 55 | 55 | 60 | 70 |
| 31 to 50 yrs | 55 | 55 | 60 | 70 |
| 50 to 71 yrs | 55 | 55 | ||
| Above 71 yrs | 55 | 55 |
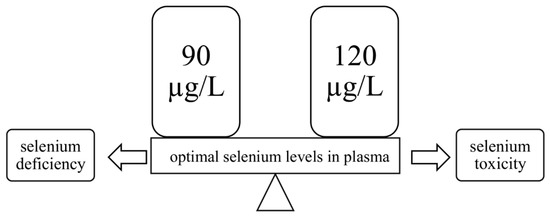
2. Absorption of Se and Its Transport in the Body
3. Selenium Deficiency
4. Selenium Overdose
References
- Burk, R.F. Recent developments in trace element metabolism and function: Newer roles of selenium in nutrition. J. Nutr. 1989, 119, 1051–1054.
- Combs, G.F., Jr. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547.
- Fordyce, F. Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology: Impacts of the Natural Environment on Public Health; Selinus, O., Alloway, B., Centeno, J.A., Finkelman, R.B., Fuge, R., Lindh, U., Smedley, P., Eds.; Elsevier: London, UK, 2005; pp. 373–415.
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; The National Academies Press: Washington, DC, USA, 2000; p. 528.
- Stockler-Pinto, M.B.; Lobo, J.; Moraes, C.; Leal, V.O.; Farage, N.E.; Rocha, A.V.; Boaventura, G.T.; Cozzolino, S.M.; Malm, O.; Mafra, D. Effect of Brazil nut supplementation on plasma levels of selenium in hemodialysis patients: 12 months of follow-up. J. Ren. Nutr. 2012, 22, 434–439.
- Pedrero, Z.; Madrid, Y. Novel approaches for selenium speciation in foodstuffs and biological specimens: A review. Anal. Chim. Acta 2009, 634, 135–152.
- Finley, J.W.; Ip, C.; Lisk, D.J.; Davis, C.D.; Hintze, K.J.; Whanger, P.D. Cancer-protective properties of high-selenium broccoli. J. Agric. Food Chem. 2001, 49, 2679–2683.
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S.
- Cappon, C.J.; Smith, J.C. Chemical form and distribution of mercury and selenium in edible seafood. J. Anal. Toxicol. 1982, 6, 10–21.
- Thiry, C.; Ruttens, A.; De Temmerman, L.; Schneider, Y.-J.; Pussemier, L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012, 130, 767–784.
- Smrkolj, P.; Pograjc, L.; Hlastan-Ribic, C.; Stibilj, V. Selenium content in selected Slovenian foodstuffs and estimated daily intakes of selenium. Food Chem. 2005, 90, 691–697.
- Bierla, K.; Dernovics, M.; Vacchina, V.; Szpunar, J.; Bertin, G.; Lobinski, R. Determination of selenocysteine and selenomethionine in edible animal tissues by 2D size-exclusion reversed-phase HPLC-ICP MS following carbamidomethylation and proteolytic extraction. Anal. Bioanal. Chem. 2008, 390, 1789–1798.
- Waegeneers, N.; Thiry, C.; De Temmerman, L.; Ruttens, A. Predicted dietary intake of selenium by the general adult population in Belgium. Food Addit. Contam. Part A 2013, 30, 278–285.
- Pappa, E.C.; Pappas, A.C.; Surai, P.F. Selenium content in selected foods from the Greek market and estimation of the daily intake. Sci. Total Environ. 2006, 372, 100–108.
- Lobinski, R.; Edmonds, J.; Suzuki, K.; Uden, P. Species-selective determination of selenium compounds in biological materials. Pure. Appl. Chem. 2000, 72, 447–461.
- Guo, X.; Wu, L. Distribution of free seleno-amino acids in plant tissue of Melilotus indica L. grown in selenium-laden soils. Ecotoxicol. Environ. Saf. 1998, 39, 207–214.
- Hoac, T.; Lundh, T.; Önning, G.; Åkesson, B. Selenoproteins and Selenium Speciation in Food. In Selenoproteins and Mimics; Springer: Berlin/Heidelberg, Germany, 2012; pp. 183–206.
- Gawor, A.; Ruszczynska, A.; Czauderna, M.; Bulska, E. Determination of Selenium Species in Muscle, Heart, and Liver Tissues of Lambs Using Mass Spectrometry Methods. Animals 2020, 10, 808.
- Clausen, J.; Nielsen, S.A. Comparison of whole blood selenium values and erythrocyte glutathione peroxidase activities of normal individuals on supplementation with selenate, selenite, L-selenomethionine, and high selenium yeast. Biol. Trace Elem. Res. 1988, 15, 125–138.
- Thomson, C.D.; Robinson, M.F.; Butler, J.A.; Whanger, P.D. Long-term supplementation with selenate and selenomethionine: Selenium and glutathione peroxidase (EC 1.11.1.9) in blood components of New Zealand women. Br. J. Nutr. 1993, 69, 577–588.
- Richie, J.P., Jr.; Muscat, J.E.; Ellison, I.; Calcagnotto, A.; Kleinman, W.; El-Bayoumy, K. Association of selenium status and blood glutathione concentrations in blacks and whites. Nutr. Cancer 2011, 63, 367–375.
- Skalny, A.V.; Burtseva, T.I.; Salnikova, E.V.; Ajsuvakova, O.P.; Skalnaya, M.G.; Kirichuk, A.A.; Tinkov, A.A. Geographic variation of environmental, food, and human hair selenium content in an industrial region of Russia. Environ. Res. 2019, 171, 293–301.
- Hintze, K.J.; Lardy, G.P.; Marchello, M.J.; Finley, J.W. Areas with high concentrations of selenium in the soil and forage produce beef with enhanced concentrations of selenium. J. Agric. Food Chem. 2001, 49, 1062–1067.
- Johnson, C.C.; Fordyce, F.M.; Rayman, M.P. Symposium on ‘Geographical and geological influences on nutrition’ Factors controlling the distribution of selenium in the environment and their impact on health and nutrition: Conference on ‘Over-and undernutrition: Challenges and approaches’. Proc. Nutr. Soc. 2010, 69, 119–132.
- Manzanares, W.; Hardy, G. Can dietary selenium intake increase the risk of toxicity in healthy children? Nutrition 2016, 32, 149–150.
- Strand, T.A.; Lillegaard, I.T.L.; Frøyland, L.; Haugen, M.; Henjum, S.; Løvik, M.; Stea, T.; Holvik, K. Assessment of Selenium Intake in Relation to Tolerable Upper Intake Levels. Eur. J. Nutr. Food Saf. 2018, 8, 155–156.
- Berntssen, M.H.G.; Betancor, M.; Caballero, M.J.; Hillestad, M.; Rasinger, J.; Hamre, K.; Sele, V.; Amlund, H.; Ørnsrud, R. Safe limits of selenomethionine and selenite supplementation to plant-based Atlantic salmon feeds. Aquaculture 2018, 495, 617–630.
- Lee, S.; Nambi, R.W.; Won, S.; Katya, K.; Bai, S.C. Dietary selenium requirement and toxicity levels in juvenile Nile tilapia, Oreochromis niloticus. Aquaculture 2016, 464, 153–158.
- Han, D.; Xie, S.; Liu, M.; Xiao, X.; Liu, H.; Zhu, X.; Yang, Y. The effects of dietary selenium on growth performances, oxidative stress and tissue selenium concentration of gibel carp (Carassius auratus gibelio). Aquac. Nutr. 2011, 17, e741–e749.
- Hurst, R.; Collings, R.; Harvey, L.J.; King, M.; Hooper, L.; Bouwman, J.; Gurinovic, M.; Fairweather-Tait, S.J. EURRECA—Estimating Selenium Requirements for Deriving Dietary Reference Values. Crit. Rev. Food Sci. Nutr. 2013, 53, 1077–1096.
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199.
- Kazi Tani, L.S.; Dennouni-Medjati, N.; Toubhans, B.; Charlet, L. Selenium Deficiency—From Soil to Thyroid Cancer. Appl. Sci. 2020, 10, 5368.
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241.
- Baum, M.K.; Shor-Posner, G.; Lai, S.; Zhang, G.; Lai, H.; Fletcher, M.A.; Sauberlich, H.; Page, J.B. High Risk of HIV-Related Mortality Is Associated with Selenium Deficiency. J. Acquir. Immune Defic. Syndr. 1997, 15, 370–374.
- Lubiński, J.; Marciniak, W.; Muszynska, M.; Jaworowska, E.; Sulikowski, M.; Jakubowska, A.; Kaczmarek, K.; Sukiennicki, G.; Falco, M.; Baszuk, P.; et al. Serum selenium levels and the risk of progression of laryngeal cancer. PLoS ONE 2018, 13, e0184873.
- Cui, Z.; Liu, D.; Liu, C.; Liu, G. Serum selenium levels and prostate cancer risk: A MOOSE-compliant meta-analysis. Medicine 2017, 96, e5944.
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium Exposure and Cancer Risk: An Updated Meta-analysis and Meta-regression. Sci. Rep. 2016, 6, 19213.
- Talebi, S.S.; Badfar, G.; Shohani, M.; Soleymani, A.; Azami, M. The Relationship between Selenium and Lung Cancer: An Updated Systematic Review and Meta-Analysis. Int. J. Cancer Manag. 2018, 11, e8370.
- Babaknejad, N.; Sayehmiri, F.; Sayehmiri, K.; Rahimifar, P.; Bahrami, S.; Delpesheh, A.; Hemati, F.; Alizadeh, S. The relationship between selenium levels and breast cancer: A systematic review and meta-analysis. Biol. Trace Elem. Res. 2014, 159, 1–7.
- Amaral, A.F.; Cantor, K.P.; Silverman, D.T.; Malats, N. Selenium and bladder cancer risk: A meta-analysis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2407–2415.
- Gong, H.Y.; He, J.G.; Li, B.S. Meta-analysis of the association between selenium and gastric cancer risk. Oncotarget 2016, 7, 15600–15605.
- Mark, S.D.; Qiao, Y.L.; Dawsey, S.M.; Wu, Y.P.; Katki, H.; Gunter, E.W.; Fraumeni, J.F., Jr.; Blot, W.J.; Dong, Z.W.; Taylor, P.R. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J. Natl. Cancer Inst. 2000, 92, 1753–1763.
- Steevens, J.; van den Brandt, P.A.; Goldbohm, R.A.; Schouten, L.J. Selenium status and the risk of esophageal and gastric cancer subtypes: The Netherlands cohort study. Gastroenterology 2010, 138, 1704–1713.
- Shen, F.; Cai, W.-S.; Li, J.-L.; Feng, Z.; Cao, J.; Xu, B. The Association Between Serum Levels of Selenium, Copper, and Magnesium with Thyroid Cancer: A Meta-analysis. Biol. Trace Elem. Res. 2015, 167, 225–235.
- Köhrle, J. Pathophysiological relevance of selenium. J. Endocrinol. Investig. 2013, 36, 1–7.
- Kieliszek, M. Selenium–Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298.
- Kiremidjian-Schumacher, L.; Roy, M.A.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Supplementation with selenium and human immune cell functions. Biol. Trace Elem. Res. 1994, 41, 115.
- Kang, D.; Lee, J.; Wu, C.; Guo, X.; Lee, B.J.; Chun, J.-S.; Kim, J.-H. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp. Mol. Med. 2020, 52, 1198–1208.
- Bügel, S.; Larsen, E.H.; Sloth, J.J.; Flytlie, K.; Overvad, K.; Steenberg, L.C.; Moesgaard, S. Absorption, excretion, and retention of selenium from a high selenium yeast in men with a high intake of selenium. Food Nutr. Res. 2008, 52, 1642.
- Mehdi, Y.; Hornick, J.-L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311.
- Combs, G.F., Jr. Biomarkers of selenium status. Nutrients 2015, 7, 2209–2236.
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228.
- Chen, J. An original discovery: Selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac. J. Clin. Nutr. 2012, 21, 320–326.
- Sun, Y.; Gao, C.; Wang, X.; Liu, Y. Preliminary quantitative proteomics analysis in chronic and latent Keshan disease by iTRAQ labeling approach. Oncotarget 2017, 8, 105761–105774.
- Zha, X.; Gao, X. Ecological analysis of Kashin-Beck osteoarthropathy risk factors in Tibet’s Qamdo City, China. Sci. Rep. 2019, 9, 2471.
- Guo, X.; Ma, W.J.; Zhang, F.; Ren, F.L.; Qu, C.J.; Lammi, M.J. Recent advances in the research of an endemic osteochondropathy in China: Kashin-Beck disease. Osteoarthr. Cartil. 2014, 22, 1774–1783.
- Schepman, K.; Engelbert, R.H.; Visser, M.M.; Yu, C.; de Vos, R. Kashin Beck Disease: More than just osteoarthrosis: A cross-sectional study regarding the influence of body function-structures and activities on level of participation. Int. Orthop. 2011, 35, 767–776.
- Mistry, H.D.; Broughton Pipkin, F.; Redman, C.W.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30.
- Fitzpatrick, A.M.; Jones, D.P.; Brown, L.A. Glutathione redox control of asthma: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 17, 375–408.
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203.
- Ellwanger, J.H.; Franke, S.I.; Bordin, D.L.; Prá, D.; Henriques, J.A. Biological functions of selenium and its potential influence on Parkinson’s disease. An. Acad. Bras. Cienc. 2016, 88, 1655–1674.
- Conner, T.S.; Richardson, A.C.; Miller, J.C. Optimal Serum Selenium Concentrations Are Associated with Lower Depressive Symptoms and Negative Mood among Young Adults. J. Nutr. 2014, 145, 59–65.
- Muzembo, B.A.; Ngatu, N.R.; Januka, K.; Huang, H.-L.; Nattadech, C.; Suzuki, T.; Wada, K.; Ikeda, S. Selenium supplementation in HIV-infected individuals: A systematic review of randomized controlled trials. Clin. Nutr. ESPEN 2019, 34, 1–7.
- Lim, T.K. Lecythis ollaria. In Edible Medicinal and Non Medicinal Plants: Volume 3, Fruits; Springer: Dordrecht, The Netherlands, 2012; pp. 138–140.
- Zwolak, I.; Zaporowska, H. Selenium interactions and toxicity: A review. Selenium interactions and toxicity. Cell Biol. Toxicol. 2012, 28, 31–46.
- Rayman, M.P.; Winther, K.H.; Pastor-Barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of long-term selenium supplementation on mortality: Results from a multiple-dose, randomised controlled trial. Free Radic. Biol. Med. 2018, 127, 46–54.
- Fernández-Martínez, A.; Charlet, L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Environ. Sci. Biotechnol. 2009, 8, 81–110.
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.B.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261.
- Nuttall, K.L. Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006, 36, 409–420.
- Köhrle, J.; Jakob, F.; Contempré, B.; Dumont, J.E. Selenium, the Thyroid, and the Endocrine System. Endocr. Rev. 2005, 26, 944–984.
- Kim, J.; Chung, H.S.; Choi, M.-K.; Roh, Y.K.; Yoo, H.J.; Park, J.H.; Kim, D.S.; Yu, J.M.; Moon, S. Association between Serum Selenium Level and the Presence of Diabetes Mellitus: A Meta-Analysis of Observational Studies. Diabetes Metab. J. 2019, 43, 447–460.
- Cherdwongchareonsuk, D.; Aguas, A.P.; Henrique, R.; Upatham, S.; Sousa Pereira, A. Toxic effects of selenium inhalation: Acute damage of the respiratory system of mice. Hum. Exp. Toxicol. 2003, 22, 551–557.




