Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nutrition & Dietetics
This entry answers the question of why selenium is such an important trace element in the human diet.
- Selenium
- micronutrients
1. Selenium in the Diet
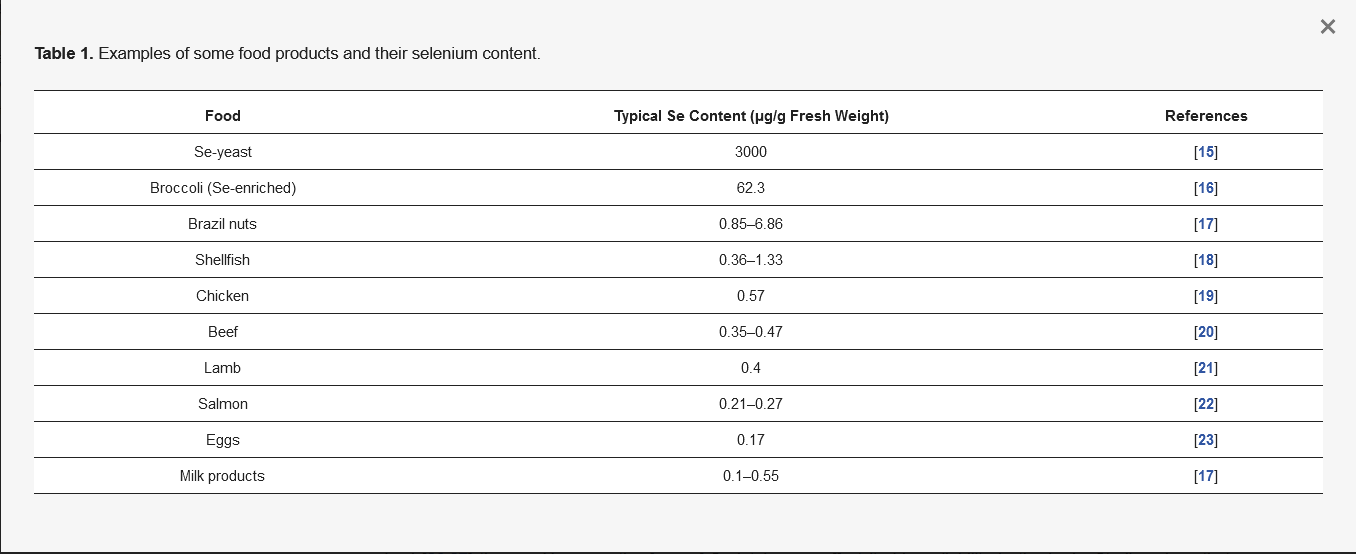
Selenium (Se) occurs as a trace element in the human body, performing a number of important biological functions, and is essential for life and health [11]. The main natural source of Se is food, but Se content varies. This is determined by the geographical location, soil quality in terms of Se concentration, and how much it is accumulated in plants. The climate and the way the food is cultivated and bred, and how we prepare it for consumption, are also important factors [3,12]. The richest sources of Se in the diet are Se-yeast (Se-enriched yeast), nuts, cereals, organ meats, fish, and seafood [13]. Brazil nuts (Bertholletia excelsa, family Lecythidaceae), a plant from the Amazon, have the highest known Se content among non-Se-enriched food [14]. Table 1 gives the Se content in some examples of food products in our diet.
Table 1. Examples of some food products and their selenium content.
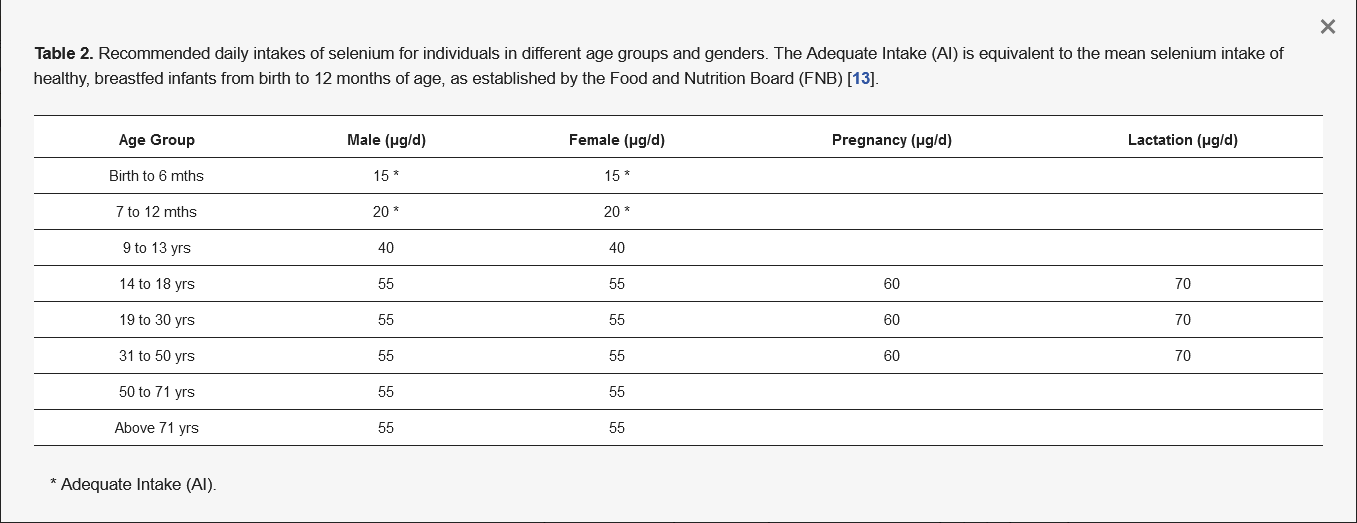
This microelement can exist in food in both chemical forms—as inorganic and organic compounds [24]. Mostly, Se is found in the form of organic compounds as selenomethionine (SeMet) and selenocysteine (Sec) in plant [25] and animal [26,27] tissues. However, the form of Se intake can affect its bioavailability in the body. Studies show that organic Se (e.g., SeMet) is more bioavailable than its inorganic compounds such as selenates or selenites [27,28,29]. Interestingly, the bioavailability of this element in the body is also modified by race. Richie et al. observed that in whites it is significantly higher than in blacks [30]. Nevertheless, the most important thing is a healthy and balanced diet that contains all the nutrients at the right levels. As far as Se and its recommended daily dose are concerned, it depends on the local content of this element in the cropland, as this affects its content in the food [31,32,33]. According to World Health Organization (WHO) standards, the recommended daily dose of Se for adults is 55 µg/day [13,34,35], while the maximum tolerable adult intake without side effects is set at 400 µg/day [13]. Of course, the requirement for Se depends on age and gender [13], as shown in Table 2.
Table 2. Recommended daily intakes of selenium for individuals in different age groups and genders. The Adequate Intake (AI) is equivalent to the mean selenium intake of healthy, breastfed infants from birth to 12 months of age, as established by the Food and Nutrition Board (FNB) [13].
It is very important to maintain an adequate level of Se—both deficiency and excess can be dangerous for human health. What is important is that this microelement has a narrow range of safety [36,37,38], so one should ensure its optimal level in the body. For biomarkers of selenium status in the body, measuring the activity of selenoproteins such as glutathione peroxidase 3 (Gpx3) and selenoprotein P (SelP, Sepp1) in plasma proves to be valuable because a decrease of their activity indicates directly a deficiency of this trace element [39,40,41]. The optimal values of Se in plasma have been estimated at 90–120 µg/L (Figure 1), which is sufficient to saturate selenoproteins in this liquid blood component [40]. The limit below which a deficiency of this element is found is set at 85 µg/L [5,42], while in Poland, its concentration in blood plasma is below this value and is about 50–55 µg/L [43], which would suggest deficiencies in the inhabitants of this country. It is worth emphasizing the fact that Cui et al. [44] observed that low serum Se concentration is associated with a higher risk of prostate cancer. Additionally, there are reports that Se also plays a key role in the prevention of other cancers [45], i.e., lung [46], breast [47], bladder [48], gastric [49,50,51], thyroid [41,52,53], and esophageal [50,51], and at a dose of 100 to 200 µg/day reduces genetic damage [1]. Furthermore, in 1994, a clinical trial conducted by Kiremidjian-Schumacher et al. [54] concluded that sodium selenite (Na2SeO3) supplementation at a dose of 200 µg/day for eight weeks significantly increased the activity of T lymphocytes and NK cells.
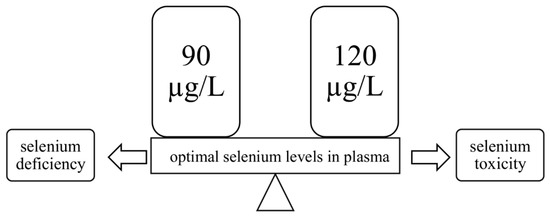
Figure 1. The range of normal (optimal) plasma selenium levels. Values above and below this range indicate selenium toxicity or deficiency, respectively.
2. Absorption of Se and Its Transport in the Body
As mentioned earlier, Se is taken with the diet in the form of organic (SeMet and Sec) and inorganic compounds (selenites, selenides) [55]. The absorption of these compounds mostly occurs in the duodenum and cecum, and their absorption mechanism depends on the chemical form of ingested Se. Inorganic compounds are absorbed by simple diffusion (selenites) or by secondary active transport, the so-called cotransport (selenides). In turn, organic compounds (SeMet, Sec, methylselenocysteine (MSC)) are absorbed by active transport in the same way as amino acids. For example, SeMet is absorbed via active transport using a sodium-dependent pump similarly to amino acids such as methionine [56,57].
After absorption of selenite and other Se compounds into the bloodstream, their rapid and selective uptake by red blood cells occurs. Subsequently, organic Se compounds undergo a reaction involving γ- or β-lyase, and selenite is reduced by glutathione (GSH) and glutathione reductase (GR). These reactions generate selenide, which is metabolized by selenophosphate synthetase 2 to selenophosphate—in this form, Se is transported to the liver [57,58]. After Se uptake by the liver, it is included in the synthesis of Sepp1 [55,58]. Sepp1 is the selenoprotein responsible for the transport of Se to peripheral tissues and organs because it has many Sec residues [57,58]. Additionally, there are also reports that Se can bind to low and very low density lipoproteins (LDL and VLDL) and α- and β-globulins and can also be transported in this way [57].
3. Selenium Deficiency
The problem of Se deficiency affects about 0.5–1 billion people in the world due to its insufficient consumption. This factor depends on the geographical area and correlates with the low content of this microelement in the soil. Regions with low soil Se content, include parts of the Congo, large parts of China, central Serbia, and, before 1984, Finland, which currently uses Se-enriched fertilizers [59]. The best known endemic diseases caused by Se deficiency, otherwise known as “geochemical diseases”, are Keshan and Kashin-Beck. The first cases of Keshan disease (KD) were discovered over 80 years ago in Keshan County (Heilongjiang Province, north-eastern China) [60]. The disease was characterized by congestive cardiomyopathy and affected children aged 2–7 years and women of reproductive age [61], whereas Kashin-Beck disease (KBD) occurs most frequently in Tibet [62] and begins in childhood around age five [63,64]. KBD is characterized by osteoarthritis, leading to the degradation of cartilage in the upper and lower limbs [63]. A too low level of this microelement in the body is also responsible for infertility in men, impaired fetal development [65], and increased risk of suffering from asthma (in the case of asthma, this is associated with a reduction in antioxidant defense, among other things, and a decrease in Gpx activity) [66]. There is also evidence that Se deficiency weakens the immune system [2] or the proper functioning of the nervous system [67]. Conner et al. [68], in their clinical study, observed that too-low Se levels in serum were associated with worse mood and increased depressive symptoms in young adults. For many years, scientists have been searching for answers as to whether Se affects the HIV viral load and AIDS progression. In 2019, a systematic review of RCTs was published, which concluded that Se has no effect on suppressing or reducing the viral load of HIV, but that there is clinical evidence that it is possible to prolong AIDS progression by supplementation with this element. Despite this, no definitive conclusions can be drawn due to their heterogeneity [69].
4. Selenium Overdose
Se poisoning occurs very rarely and is the result of excessive supplementation or a diet rich in products with a high content of this microelement. The consumption of “Coco de Mono” (Lecythis ollaria) nuts, which accumulate huge amounts of Se (7–12 g Se/kg of dry matter), caused acute Se poisoning in Venezuela [70]. The excess Se intake was associated mainly with the geographical area, whose soils are characterized by its high content [71]. An example of such dependence is Enshi (Hubei Province, South China), where there is a high content of Se in the soil—the inhabitants of this area had symptoms of the toxic effects of Se on the body because they had a dietary intake of over 850 µg Se/day [13]. On the other hand, it is also worth noting that taking lower doses of Se in the order of 300 µg/day may have adverse effects on the body. In the Danish Prevention of Cancer by Intervention with Selenium (PRECISE) trial, it was observed that long-term intake of 300 µg Se/day in the form of Se-enriched yeast increased mortality in the study population. For this reason, such a high daily supplementation or intake of Se should be avoided [72].
The toxicity of this element depends on many factors—its chemical form, ingested dose, interactions with other dietary components, and the physiological condition of the body [73]. As far as Se compounds are concerned, its inorganic forms exhibit higher toxicity than when they are in the form of organic compounds. This is because inorganic Se (selenides, selenites) has a prooxidant effect on thiols (GSH), producing free oxygen radicals, while methylated forms (organic) are less toxic due to their easier excretion [19]. Symptoms of acute Se poisoning are rather non-specific and therefore may cause problems in diagnosis. These include hypotension, rapid heartbeat (tachycardia), neurological disorders, fever, dry cough, and pulmonary edema. In both cases of toxicity—acute and chronic—anemia, gastrointestinal disorders, salivation, and blindness occur. In contrast, chronic Se toxicity, otherwise known as selenosis, is characterized by nail fragility, hair loss, skin lesions, joint pain, tooth decay, and a specific garlic odor in the exhaled breath (presence of volatile compound—dimethyl selenide) [13,74,75]. Excessive doses of Se also cause endocrine disorders in the synthesis of thyroid hormones, growth hormones (GH), and insulin-like growth factors (IGF-1) [76]. It is also worth emphasizing the link between Se and type 2 diabetes mellitus (T2DM). A meta-analysis of the observational studies conducted by Kim et al. in 2019 [77] showed that increased Se intake increases the risk of T2DM. Very importantly, this meta-analysis found that the odds of developing T2DM in people with high Se levels are approximately twofold higher than the odds in people with lower or optimal levels of this trace element. Se is not only found in the soil, water, and food; poisoning with its compounds may also occur as a result of inhalation (e.g., highly toxic H2Se), so its maximum concentration in the air should be less than 0.2 mg/m3. Symptoms of acute inhalation intoxication are chemical pneumonia, lung hemorrhage and edema, and bronchiolitis, as well as extrapulmonary effects—nausea, headaches, and eye irritation [15,78].
This entry is adapted from the peer-reviewed paper 10.3390/nu13051649
This entry is offline, you can click here to edit this entry!
