
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Heike Allgayer | + 2184 word(s) | 2184 | 2021-04-26 05:05:51 | | | |
| 2 | Rita Xu | -9 word(s) | 2175 | 2021-04-27 06:21:15 | | |
Video Upload Options
The role and function of bacteriophages (phages) has been underestimated so far. Natural compounds such as essential oils and tea have been used successfully in naturopathy and folk medicine for hundreds of years. Current research is unveiling the molecular role of their antibacterial, anti-inflammatory, and anticancer properties. The current interdisciplinary review summarizes current knowledge on dietary compounds as to their capacity to modulate the activity of phages, thus potentially contributing to (the modulation of) several gastrointestinal diseases, such as (chronic) inflammation, and even cancer.
1. Introduction
The impact of the gut bacteriome on the human physiology is currently being investigated and seems to have a significant influence on the development and treatment of various diseases. Collectively, the over one thousand bacterial species residing in the human gut encode 3.3 million genes, expanding the human genome 150 times over [1]. Several studies have demonstrated that microorganisms present in the human gut (the gut microbiome) modulate human physiology at different levels. Intestinal bacteria not only metabolize polysaccharides that would be otherwise indigestible [2], but also regulate peristalsis [3], help to keep a proper intestinal morphology as it has been shown in a gnotobiotic pig model [4], maintain the integrity of the intestinal barrier [5][6][7], attenuate inflammation [8][9], reduce the virulence of pathogenic species [10], and even influence the action of anticancer drugs [11]. Although it has been proposed to consider the intestinal microorganisms as symbionts rather than simple commensal species [12], our understanding of the dynamics underlying the interactions between host and gut microbiome is still limited [13][14].
Bacteriophages (or phages for short) represent a significant modulator of the gut microbiome [15]. By definition, phages infect bacteria, but more and more data highlight the interrelation between eukaryotic cells and bacterial viruses. Phages can interact directly with the human body since they can translocate inside eukaryotic cells [16] and activate the immune system, exacerbating ongoing colitis symptoms and boosting the antibacterial response [17]. It has recently been proposed to consider phages as human pathogens [18].
In the last few years, phages have become a crucial topic in the medical and microbiological fields because these viruses can be used as a treatment of bacterial infections in the context of the rising problem of antibiotic resistance [19][20][21]. As our understanding of phage biology increases, the applications of phage therapy also expand. Phages have been applied to treat bacterial infections ever since their discovery, and phage therapy is becoming more and more popular in fields ranging from dentistry to medical microbiology [22][23][24][25]. For example, phages are currently being evaluated to fight infections in poultry that are still an economic and health issue [24]. Recent studies suggest that phages can also be applied in antiviral and anticancer therapies. For instance, it has been proposed that phage T4 might be used as a co-treatment for COVID-19 because this phage reduces the immune response, which is an important contributor to the fatality associated with this disease [26]. Furthermore, it has been shown that phages bind to cancerous cells and reduce the size of the tumor mass in different mouse models [17][27][28], opening the possibility of phage-mediated oncolytic virotherapy.
Diet can influence the gut microbiome, and it is actively used as an intervention to reduce the risk of developing diseases [29]. Particular components have been shown to be of benefit in the treatment of even severe disease conditions up to cancer. For example, in own previous studies, it was demonstrated that the plant-derivatives curcumin and artesunate inhibit tumor cell invasion and metastasis, at least in part via regulating the expression of proteolytic enzymes, the molecular cascades involving transcriptional factors and microRNAs, respectively [30][31][32][33]. However, there is a lack of studies describing how dietary compounds impact microorganisms in general and phages in particular. Seminal studies in the 1950 s demonstrated the antiviral activity of tannins, which are contained in popular beverages such as tea and coffee, and of acerin, the active component of maple fruit [34][35]. Especially, tea showed broad antimicrobial activity, including inactivation of phages [36]. It is also known that essential oils have antibacterial and antiviral properties as well as anti-inflammatory and regenerative activities [37]. Nevertheless, gaining experimental knowledge on the influence of dietary compounds on phages as modulators of microbiota has not yet been in focus of attention in the research community.
Most of the studies related to the effect of dietary compounds on phages have been focusing on human viruses associated with gastroenteritis. Phages have been used as surrogates for viruses that cannot be easily cultivated, such as norovirus, rather than for studying bacterial virus biology as such. Also, most of the bacteriophage studies so far have been limited to phages infecting Escherichia coli (coliphages). Nonetheless, E. coli plays an essential role in human health since certain strains of this species, known as Shiga toxin-producing E. coli (STEC), are widespread food-borne pathogens. The most prevalent STECs are O157, O26, O45, O103, O111, O121, and O145. These seven serotypes induce diseases ranging from acute diarrhea to hemorrhagic colitis and fatal hemolytic syndrome [38][39][40].
The main STEC derived virulence factor is shiga-like toxin (Stx), which is encoded by the prophages 933 J (Stx1) and 933 W (Stx2) [41][42]. Upon activation, these prophages express Stx, and they can horizontally spread this gene by transduction [43][44]. Genotoxins, such as cytolethal distending toxins and colibactin, are considered cancer risk factors and can be found in pathogenic E. coli strains [45]. Interestingly, many natural compounds have been shown to be bactericidal against pathogens [46], and to suppress the biological activity of toxins, including the cholera and ricin toxins [46][47][48][49]. Peas showed to bind with high efficiency Stx, acting as toxic-scavengers, whereas beans can reduce the intake of Stx [50].
2. Interactions between Phages and Bacteria in the Gut Microbiome
Phages were first described by the French-Canadian Félix d’Hérelle, of Institute Pasteur in 1917, who also defined the term ‘bacteriophage’ (“eater of bacteria”). As a first, pioneering phage-based therapy, he applied bacteriophages to treat Shigella infections in soldiers, establishing what became known as phage therapy [51][52][53]. Phages can be subdivided into two groups: virulent (lytic) and temperate (lysogenic) (Figure 1) [54]. Lytic viruses start the replication process soon after the infection of the bacterial host. Once the progeny virions have assembled in a sufficient number (the burst size), the cell bursts open, releasing the new phages in the surrounding environment. Lysogenic phages have an additional phase: they can integrate as prophages in the bacterial chromosome and undergo a latency period where only a viral transcription suppressor is produced actively. In particular contexts, such as bacterial starvation or DNA damage, the suppression control is relieved, and the prophage enters the lytic phase. Conversely, in the presence of a high number of infected bacteria, phages exit the lytic phase and initiate lysogeny [55]. Both virulent and temperate phages modulate the bacterial population through lysis.
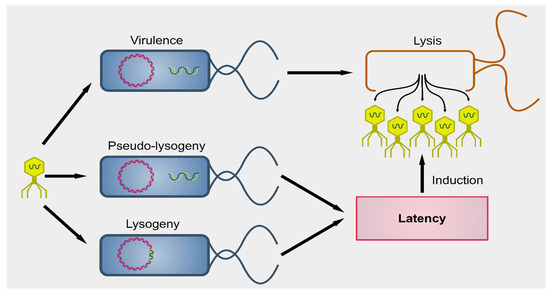
Figure 1. Outcomes of phagial infection of bacteria. A virulent phage (yellow particle on the left) can infect a bacterium (in blue). The replication of the phage leads to lysis of the host cell, releasing the viral progeny (yellow particles on the right). Alternatively, some viral species known as temperate can establish an additional step known as latency. The phagial genome can remain independent from that of the bacterium (pseudo-lysogeny) or become integrated into the host’s genome (lysogeny). In both cases, the viral expression is kept to a minimum and there is no virion production until several cellular conditions are met. Upon induction, temperate phages enter the lytic pathway and determine the lysis of the host.
Phages can also modulate the bacterial population, indirectly. It is well known that bacteria must undergo a fierce competition within each ecological niche, and, therefore, some species have developed virulence factors to improve their chances of survival [56]. Moreover, the microbial competition is complex and difficult to predict. For instance, Lactobacillus delbrueckii and L. rhamnosus inhibit E. coli O157, but L. plantarum suppresses the commensal strains of E. coli but not O157, and L. paracasei does not constrain E. coli at all [57]. In addition, the suppression of one species might cause the unexpected expansion of a species not apparently associated with the suppressed one. For instance, E. coli fosters the growth of B. fragilis but represses B. vulgatus. Knocking down E. coli by phage T4 is, therefore, followed by a contraction of the prevalence of B. fragilis and an increased growth of B. vulgatus, but also of Proteus mirabilis and Akkermansia muciniphila [58]. It is also known that commensal species can neutralize toxins, reducing the fitness of the pathogens. For instance, surface proteins of L. plantarum can neutralize Stx, reducing the cytotoxicity (and, thus, the fitness) of E. coli O157 [59]. Therefore, the alteration of even one species due to phagial predation can have drastic consequences for the microbiome.
Mounting evidence suggests that phages have access to eukaryotic (and human) cells [60]. Even though tissues are expected to be sterile, it has been known for decades that an ingestion of phage preparations during phage therapy is followed by a recovery of phages in human urine and blood within a few minutes from the administration [61][62]. This recovery implies that the viruses had somehow crossed the gastrointestinal barrier. Recent virome studies have identified genes belonging to phages in both blood and brain [63][64]. The circulation of phages in the peripheral blood has been named ‘phagemia’, but there is a lack of hard evidence for its actual existence in physiological conditions [65]. Furthermore, phages can be actively transported from one side to another of intestinal cells (transcytosis) via the Golgi network [16].
3. Effect of Dietary Compounds on Phages
Several dietary compounds can alter the physiology of phages, as summarized in Figure 2. Although many studies showed a connection between nutrition and intestinal microbiome, there are only a few studies that deal with the effects of nutrition on the activity of phages. Seminal work in the 1960 s indicated that amino acids and vitamins had a different impact on the induction of prophage λ in E. coli [66]. For instance, the amino acid cysteine was an inducer, but its oxidized derivative cystine was not. About four decades later, it was shown that essential oils extracted from chamomile, lemongrass, cinnamon, and geranium could greatly reduce the infectivity of E. coli T7 and S. aureus SA, whereas others (such as angelica, cardamom, lime, and rosemary) affected only the former phage [67]. A recent study reported how different compounds could selectively activate some viruses but not others in bacterial growth and prophage-induction assays [68]. This study demonstrated how stevia, a natural sweetener obtained from the Brazilian shrub Stevia rebaudiana [69], could strongly induce prophages present in Bacteroides thetaiotamicron and Staphylococcus aureus but not in Enterococcus faecalis, whereas uva ursi (derived from the shrub plant Arctostaphylos uva-ursi), aspartame (a peptide), and propolis (a flavonoid) resulted in the opposite. These data indicate that dietary compounds can modulate the gut virome and, consequently, alter the gut bacteriome.
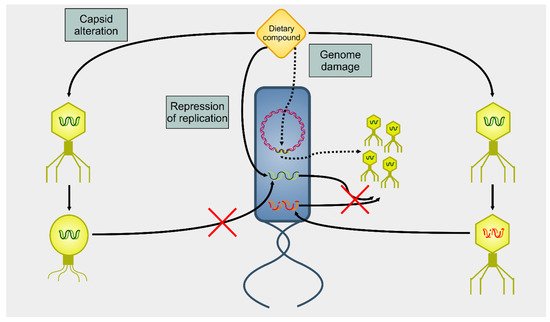
Figure 2. Summary of actions on phages on dietary compounds. There are three main mechanisms of action of dietary compounds upon phages. A dietary compound can modify the capsid, blocking the infectivity of the targeted phage (capsid alteration). Alternatively, dietary compounds can lead to the degradation of nucleic acids (genome damage). In this case, a phage can infect the host, but there will be neither lysis nor viral progeny. However, DNA damage to the host cell’s genome triggers the induction of prophages (dotted arrows). A final mechanism of action (repression of replication) involves an interference with the replication of the viral genome. Even in this case, there is infection, but no viral progeny is produced.
Experiments measuring the effect of dietary compounds on phage activity have been based on few classes of compounds, mainly polyphenols. These are molecules that contain one or more phenolic aromatic rings (benzenes with hydroxide moieties). Polyphenols can be subdivided into phenolic acid derivatives and flavonoids [70]. The former can, in turn, be subdivided into derivatives of either hydroxybenzoic acid (for instance, gallic acid) or cinnamic acid (for example, caffeic acid) [71]. Tea, the second most frequently consumed beverage after water, is a primary source for gallic acid [72]. Coffee, whose consumption is increasing worldwide [73], contains chlorogenic acid (a combination of caffeic acid and quinic acid) [71]. Tannic acid, which contains several hydroxybenzoic acid moieties, is particularly abundant in berries; soy is rich in isoflavonoids, such as genistein and daidzein [74]. The exact mechanism of action of these phenol-compounds is not entirely understood. Still, it is known that they can be beneficial for human physiology and have been used in folk medicine since millennia [75]. They are currently being investigated for their anticancer activity [76][77][78].
The chemical structure of the compounds discussed herein is shown in Figure 3, Figure 4 and Figure 5. A summary of the activities identified is given in Table 1. The most common outcome of exposure to a given nutrient is a loss of infectivity; this is measured by comparing the plaque-forming units (PFU) of a control and an exposed suspension (measured in mL) of phages. If the control and the exposed suspensions showed, for instance, 1010 and 109 PFU/mL, then the reduction is said to be one log10.
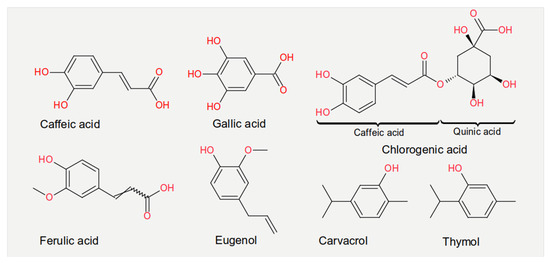
Figure 3. Chemical structures of the phenolic acids reported in the present review.
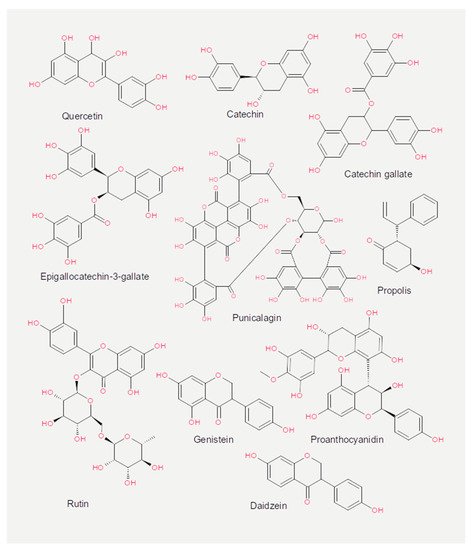
Figure 4. Chemical structures of the flavonoids reported in the present review.
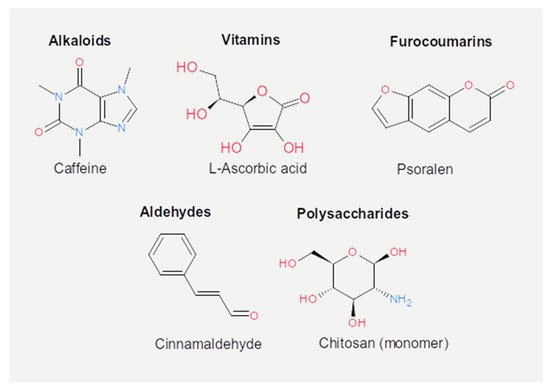
Figure 5. Chemical structures of the other active dietary compounds reported in the present review.
References
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65.
- Ramakrishnan, R.; Donahue, H.; Garcia, D.; Tan, J.; Shimizu, N.; Rice, A.P.; Ling, P.D. Epstein-barr virus BART9 miRNA modulates LMP1 levels and affects growth rate of nasal NK T cell lymphomas. PLoS ONE 2011, 6, e27271.
- Bultman, S.J. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol. Nutr. Food Res. 2017, 61, 1500902.
- Shirkey, T.W.; Siggers, R.H.; Goldade, B.G.; Marshall, J.K.; Drew, M.D.; Laarveld, B.; Kessel, A.G.V. Effects of commensal bacteria on intestinal morphology and expression of proinflammatory cytokines in the gnotobiotic pig. Exp. Biol. Med. 2006, 231, 1333–1345.
- Pull, S.L.; Doherty, J.M.; Mills, J.C.; Gordon, J.I.; Stappenbeck, T.S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA 2005, 102, 99–104.
- Ukena, S.N.; Singh, A.; Dringenberg, U.; Engelhardt, R.; Seidler, U.; Hansen, W.; Bleich, A.; Bruder, D.; Franzke, A.; Rogler, G.; et al. Probiotic escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS ONE 2007, 2, e1308.
- Mennigen, R.; Nolte, K.; Rijcken, E.; Utech, M.; Loeffler, B.; Senninger, N.; Bruewer, M. Probiotic mixture VSL # 3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am. J. Physiol. 2009, 296, 1140–1149.
- Kelly, D.; Campbell, J.I.; King, T.P.; Grant, G.; Jansson, E.A.; Coutts, A.G.P.; Pettersson, S.; Conway, S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shutting of PPAR and ReIA. Nat. Immunol. 2004, 5, 104–112.
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004, 118, 229–241.
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–549.
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillre, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976.
- Xu, J.; Gordon, J.I. Honor thy symbionts. Proc. Natl. Acad. Sci. USA 2003, 100, 10452–10459.
- Maruvada, P.; Leone, V.; Kaplan, L.M.; Chang, E.B. The Human microbiome and obesity: Moving beyond associations. Cell Host Microbe 2017, 22, 589–599.
- Beller, L.; Matthijnssens, J. What is (not) known about the dynamics of the human gut virome in health and disease. Curr. Opin. Virol. 2019, 37, 52–57.
- Sutton, T.D.S.; Hill, C. Gut bacteriophage: Current understanding and challenges. Front. Endocrinol. 2019, 10, 784.
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M.; et al. Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers. mBio 2017, 8.
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Brown, D.G.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe 2019, 25, 285–299.
- Tetz, G.; Tetz, V. Bacteriophages as new human viral pathogens. Microorganisms 2018, 6, 54.
- Levin, B.R.; Stewart, F.M.; Chao, L. Resource-limited growth, competition, and predation: A model and experimental studies with bacteria and bacteriophage. Math. Biosci. 1977, 111, 3–24.
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85.
- Barr, J.J.; Auro, R.; Furlan, M.; Whiteson, K.L.; Erb, M.L.; Pogliano, J.; Stotland, A.; Wolkowicz, R.; Cutting, A.S.; Doran, K.S.; et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 10771–10776.
- Viertel, T.M.; Ritter, K.; Horz, H.-P. Viruses versus bacteria-novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J. Antimicrob. Chemother. 2014, 69, 2326–2336.
- Shlezinger, M.; Khalifa, L.; Houri-Haddad, Y.; Coppenhagen-Glazer, S.; Resch, G.; Que, Y.-A.; Beyth, S.; Dorfman, E.; Hazan, R.; Beyth, N. Phage therapy: A new horizon in the antibacterial treatment of oral pathogens. Curr. Top. Med. Chem. 2017, 17, 1199–1211.
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179.
- Chang, R.Y.K.; Wallin, M.; Lin, Y.; Leung, S.S.Y.; Wang, H.; Morales, S.; Chan, H.K. Phage therapy for respiratory infections. Adv. Drug Deliv. Rev. 2018, 133, 76–86.
- Gorski, A.; Midzybrodzki, R.; aczek, M.; Borysowski, J. Phages in the fight against COVID-19? Future Microbiol. 2020, 15, 1095–1100.
- Dabrowska, K.; Opolski, A.; Wietrzyk, J.; Gorski, A. Anticancer activity of bacteriophage T4 and its mutant HAP1 in mouse experimental tumour models. Anticancer Res. 2004, 24, 3991–3995.
- Dabrowska, K.; Opolski, A.; Wietrzyk, J.; Switala-Jelen, K.; Boratynski, J.; Nasulewicz, A.; Lipinska, L.; Chybicka, A.; Kujawa, M.; Zabel, M.; et al. Antitumor activity of bacteriophages in murine experimental cancer models caused possibly by inhibition of beta3 integrin signaling pathway. Acta Virol. 2004, 48, 241–248.
- Voreades, N.; Kozil, A.; Weir, T.L. Diet and the development of the human intestinal microbiome. Front. Microbiol. 2014, 5, 494.
- Leupold, J.H.; Yang, H.-S.; Colburn, N.H.; Asangani, I.; Post, S.; Allgayer, H. Tumor suppressor Pdcd4 inhibits invasion/intravasation and regulates urokinase receptor (u-PAR) gene expression via Sp-transcription factors. Oncogene 2007, 26, 4550–4562.
- Asangani, I.A.; Rasheed, S.A.K.; Nikolova, D.A.; Leupold, J.H.; Colburn, N.H.; Post, S.; Allgayer, H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2008, 27, 2128–2136.
- Rasheed, S.A.K.; Efferth, T.; Asangani, I.A.; Allgayer, H. First evidence that the antimalarial drug artesunate inhibits invasion and in vivo metastasis in lung cancer by targeting essential extracellular proteases. Int. J. Cancer 2010, 127, 1475–1485.
- Mudduluru, G.; George-William, J.N.; Muppala, S.; Asangani, I.A.; Kumarswamy, R.; Nelson, L.D.; Allgayer, H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011, 31, 185–197.
- Fischer, G.; Gardell, S.; Jorpes, E. On the chemical nature of acerin and the virucidal and antiviral effects of some vegetable tannins. Experientia 1954, 10, 329–330.
- Martinek, R.G.; Wolman, W. Xanthines, tannins, and sodium in coffee, tea, and cocoa. J. Am. Med. Assoc. 1955, 158, 1031–1051.
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134.
- Kon, K.V.; Rai, M.K. Plant essential oils and their constituents in coping with multidrug-resistant bacteria. Expert Rev. Anti-Infect. Ther. 2012, 10, 775–790.
- Tarr, P.I. Escherichia coli O157:H7: Overview of clinical and epidemiological issues. J. Food Prot. 1994, 57, 632–636.
- Su, C.; Brandt, L.J. Escherichia coli O157:H7 infection in humans. Ann. Intern. Med. 1995, 123, 698–714.
- Johnson, K.E.; Thorpe, C.M.; Sears, C.L. The emerging clinical importance of non-O157 Shiga toxin-producing Escherichia coli. Clin. Infect. Dis. 2006, 43, 1587–1595.
- O’Brien, A.D.; Newland, J.W.; Miller, S.F.; Holmes, R.K.; Smith, H.W.; Formal, S.B. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 1984, 226, 694–696.
- Strockbine, N.A.; Marques, L.R.M.; Newland, J.W.; Smith, H.W.; Holmes, R.K.; O’Brien, A.D. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect. Immun. 1986, 53, 135–140.
- Johansen, B.K.; Wasteson, Y.; Granum, P.E.; Brynestad, S. Mosaic structure of Shiga-toxin-2-encoding phages isolated from Escherichia coli O157:H7 indicates frequent gene exchange between lambdoid phage genomes. Microbiology 2001, 147, 1929–1936.
- Canchaya, C.; Fournous, G.; Chibani-Chennoufi, S.; Dillmann, M.L.; Brssow, H. Phage as agents of lateral gene transfer. Curr. Opin. Microbiol. 2003, 6, 417–424.
- Taieb, F.; Petit, C.; Nougayrde, J.-P.; Oswald, E. The enterobacterial genotoxins: Cytolethal distending toxin and colibactin. EcoSal Plus 2016, 7.
- Friedman, M.; Rasooly, R. Review of the inhibition of biological activities of food-related selected toxins by natural compounds. Toxins 2013, 5, 743–775.
- Oi, H.; Matsuura, D.; Miyake, M.; Ueno, M.; Takai, I.; Yamamoto, T.; Kubo, M.; Moss, J.; Noda, M. Identification in traditional herbal medications and confirmation by synthesis of factors that inhibit cholera toxin-induced fluid accumulation. Proc. Natl. Acad. Sci. USA 2002, 99, 3042–3046.
- Saito, T.; Miyake, M.; Toba, M.; Okamatsu, H.; Shimizu, S.; Noda, M. Inhibition by apple polyphenols of ADP-ribosyltransferase activity of cholera toxin and toxin-induced fluid accumulation in mice. Microbiol. Immunol. 2002, 46, 249–255.
- Morinaga, N.; Iwamaru, Y.; Yahiro, K.; Tagashira, M.; Moss, J.; Noda, M. Differential activities of plant polyphenols on the binding and internalization of cholera toxin in vero cells. J. Biol. Chem. 2005, 280, 23303–23309.
- Becker, P.M.; van der Meulen, J.; Jansman, A.J.M.; van Wikselaar, P.G. In vitro inhibition of ETEC K88 adhesion by pea hulls and of LT enterotoxin binding by faba bean hulls. J. Anim. Physiol. Anim. Nutr. 2012, 96, 1121–1126.
- D’Herelle, F. On an invisible microbe antagonistic toward dysenteric bacilli: Brief note by Mr. F. D’Herelle, presented by Mr. Roux. Res. Microbiol. 2007, 158, 553–554.
- Chanishvili, N. Phage therapy-history from twort and d’herelle through soviet experience to current approaches. Adv. Vir. Res. 2012, 83, 3–40.
- Salmond, G.P.C.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786.
- Davies, E.V.; Winstanley, C.; Fothergill, J.L.; James, C.E. The role of temperate bacteriophages in bacterial infection. FEMS Microbiol. Lett. 2016, 363, 15.
- Erez, Z.; Steinberger-Levy, I.; Shamir, M.; Doron, S.; Stokar-Avihail, A.; Peleg, Y.; Melamed, S.; Leavitt, A.; Savidor, A.; Albeck, S.; et al. Communication between viruses guides lysis-lysogeny decisions. Nature 2017, 541, 488–493.
- Sharma, A.K.; Dhasmana, N.; Dubey, N.; Kumar, N.; Gangwal, A.; Gupta, M.; Singh, Y. Bacterial virulence factors: Secreted for survival. Indian J. Microbiol. 2017, 57, 1–10.
- Mogna, L.; Del Piano, M.; Deidda, F.; Nicola, S.; Soattini, L.; Debiaggi, R.; Sforza, F.; Strozzi, G.; Mogna, G. Assessment of the in vitro inhibitory activity of specific probiotic bacteria against different Escherichia coli strains. J. Clin. Gastroenterol. 2012, 46, S29–S32.
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe 2019, 25, 803–814.
- Kakisu, E.; Abraham, A.G.; Farinati, C.T.; Ibarra, C.; De Antoni, G.L. Lactobacillus plantarum isolated from kefir protects vero cells from cytotoxicity by type-II shiga toxin from Escherichia coli O157:H7. J. Dairy Res. 2013, 80, 64–71.
- Barr, J.J. A bacteriophages journey through the human body. Immunol. Rev. 2017, 279, 106–122.
- Caldwell, J.A. Bacteriologic and bacteriophagic study of infected urines. J. Infect. Dis. 1928, 43, 353–362.
- Weber-Dabrowska, B.; Dabrowski, M.; Slopek, S. Studies on bacteriophage penetration in patients subjected to phage therapy. Arch. Immunol. Ther. Exp. 1987, 35, 563–568.
- Moustafa, A.; Xie, C.; Kirkness, E.; Biggs, W.; Wong, E.; Turpaz, Y.; Bloom, K.; Delwart, E.; Nelson, K.E.; Venter, J.C.; et al. The blood DNA virome in 8,000 humans. PLoS Pathog. 2017, 13, 1–20.
- Ghose, C.; Ly, M.; Schwanemann, L.K.; Shin, J.H.; Atab, K.; Barr, J.J.; Little, M.; Schooley, R.T.; Chopyk, J.; Pride, D.T. The virome of cerebrospinal fluid: Viruses where we once thought there were none. Front. Microbiol. 2019, 10, 1–14.
- Gorski, A.; Wazna, E.; Dąbrowska, B.W.; Dąbrowska, K.; Świtała-Jeleń, K.; Międzybrodzki, R. Bacteriophage translocation. FEMS Immunol. Med. Microbiol. 2006, 46, 313–319.
- Heinemann, B.; Howard, A.J. Induction of lambda-bacteriophage in Escherichia coli as a screening test for potential antitumor agents. Appl. Environ. Microbiol. 1964, 12, 234–239.
- Chao, S.C.; Young, D.G.; Oberg, C.J. Screening for inhibitory activity of essential oils on selected bacteria, fungi and viruses. J. Essent. Oil Res. 2000, 12, 639–649.
- Boling, L.; Cuevas, D.A.; Grasis, J.A.; Kang, H.S.; Knowles, B.; Levi, K.; Maughan, H.; McNair, K.; Rojas, M.I.; Sanchez, S.E.; et al. Dietary prophage inducers and antimicrobials: Toward landscaping the human gut microbiome. Gut Microbes 2020, 1, 1–14.
- Goyal, S.K.; Samsher; Goyal, R.K. Stevia (Stevia rebaudiana) a bio-sweetener: A review. Int. J. Food Sci. Nutr. 2010, 61, 1–10.
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243.
- Manach, C.; Scalbert, A.; Morand, C.; Rmsy, C.; Jimnez, L. Polyphenols: Food sources and bioavailability. J. Clin. Nutr. 2004, 79, 727–747.
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195.
- Nehlig, A.; Debry, G. Potential genotoxic, mutagenic and antimutagenic effects of coffee: A review. Mutat. Res. 1994, 317, 145–162.
- Setchell, K.D.R.; Welsh, M.B.; Lim, C.K. High-performance liquid chromatographic analysis of phytoestrogens in soy protein preparations with ultraviolet, electrochemical and thermospray mass spectrometric detection. J. Chromatogr. A 1987, 386, 315–323.
- Jiang, T.A. Health benefits of culinary herbs and spices. J. AOAC Int. 2019, 102, 395–411.
- Busch, C.; Burkard, M.; Leischner, C.; Lauer, U.M.; Frank, J.; Venturelli, S. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin. Epigenetics 2015, 7, 64.
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178.
- Burkard, M.; Leischner, C.; Lauer, U.M.; Busch, C.; Venturelli, S.; Frank, J. Dietary flavonoids and modulation of natural killer cells: Implications in malignant and viral diseases. J. Nutrit. Biochem. 2017, 46, 1–12.




