The role and function of bacteriophages (phages) has been underestimated so far. Natural compounds such as essential oils and tea have been used successfully in naturopathy and folk medicine for hundreds of years. Current research is unveiling the molecular role of their antibacterial, anti-inflammatory, and anticancer properties. The current interdisciplinary review summarizes current knowledge on dietary compounds as to their capacity to modulate the activity of phages, thus potentially contributing to (the modulation of) several gastrointestinal diseases, such as (chronic) inflammation, and even cancer.
- Phage
- bacteriophages
- diet
- infection
- colorectal
- cancer
1. Introduction
The impact of the gut bacteriome on the human physiology is currently being investigated and seems to have a significant influence on the development and treatment of various diseases. Collectively, the over one thousand bacterial species residing in the human gut encode 3.3 million genes, expanding the human genome 150 times over [1]. Several studies have demonstrated that microorganisms present in the human gut (the gut microbiome) modulate human physiology at different levels. Intestinal bacteria not only metabolize polysaccharides that would be otherwise indigestible [2], but also regulate peristalsis [3], help to keep a proper intestinal morphology as it has been shown in a gnotobiotic pig model [4], maintain the integrity of the intestinal barrier [5,6,7], attenuate inflammation [8,9], reduce the virulence of pathogenic species [10], and even influence the action of anticancer drugs [11]. Although it has been proposed to consider the intestinal microorganisms as symbionts rather than simple commensal species [12], our understanding of the dynamics underlying the interactions between host and gut microbiome is still limited [13,14].
Bacteriophages (or phages for short) represent a significant modulator of the gut microbiome [15]. By definition, phages infect bacteria, but more and more data highlight the interrelation between eukaryotic cells and bacterial viruses. Phages can interact directly with the human body since they can translocate inside eukaryotic cells [16] and activate the immune system, exacerbating ongoing colitis symptoms and boosting the antibacterial response [17]. It has recently been proposed to consider phages as human pathogens [18].
In the last few years, phages have become a crucial topic in the medical and microbiological fields because these viruses can be used as a treatment of bacterial infections in the context of the rising problem of antibiotic resistance [19,20,21]. As our understanding of phage biology increases, the applications of phage therapy also expand. Phages have been applied to treat bacterial infections ever since their discovery, and phage therapy is becoming more and more popular in fields ranging from dentistry to medical microbiology [22,23,24,25]. For example, phages are currently being evaluated to fight infections in poultry that are still an economic and health issue [24]. Recent studies suggest that phages can also be applied in antiviral and anticancer therapies. For instance, it has been proposed that phage T4 might be used as a co-treatment for COVID-19 because this phage reduces the immune response, which is an important contributor to the fatality associated with this disease [26]. Furthermore, it has been shown that phages bind to cancerous cells and reduce the size of the tumor mass in different mouse models [17,27,28], opening the possibility of phage-mediated oncolytic virotherapy.
Diet can influence the gut microbiome, and it is actively used as an intervention to reduce the risk of developing diseases [29]. Particular components have been shown to be of benefit in the treatment of even severe disease conditions up to cancer. For example, in own previous studies, it was demonstrated that the plant-derivatives curcumin and artesunate inhibit tumor cell invasion and metastasis, at least in part via regulating the expression of proteolytic enzymes, the molecular cascades involving transcriptional factors and microRNAs, respectively [30,31,32,33]. However, there is a lack of studies describing how dietary compounds impact microorganisms in general and phages in particular. Seminal studies in the 1950 s demonstrated the antiviral activity of tannins, which are contained in popular beverages such as tea and coffee, and of acerin, the active component of maple fruit [34,35]. Especially, tea showed broad antimicrobial activity, including inactivation of phages [36]. It is also known that essential oils have antibacterial and antiviral properties as well as anti-inflammatory and regenerative activities [37]. Nevertheless, gaining experimental knowledge on the influence of dietary compounds on phages as modulators of microbiota has not yet been in focus of attention in the research community.
Most of the studies related to the effect of dietary compounds on phages have been focusing on human viruses associated with gastroenteritis. Phages have been used as surrogates for viruses that cannot be easily cultivated, such as norovirus, rather than for studying bacterial virus biology as such. Also, most of the bacteriophage studies so far have been limited to phages infecting Escherichia coli (coliphages). Nonetheless, E. coli plays an essential role in human health since certain strains of this species, known as Shiga toxin-producing E. coli (STEC), are widespread food-borne pathogens. The most prevalent STECs are O157, O26, O45, O103, O111, O121, and O145. These seven serotypes induce diseases ranging from acute diarrhea to hemorrhagic colitis and fatal hemolytic syndrome [38,39,40].
The main STEC derived virulence factor is shiga-like toxin (Stx), which is encoded by the prophages 933 J (Stx1) and 933 W (Stx2) [41,42]. Upon activation, these prophages express Stx, and they can horizontally spread this gene by transduction [43,44]. Genotoxins, such as cytolethal distending toxins and colibactin, are considered cancer risk factors and can be found in pathogenic E. coli strains [45]. Interestingly, many natural compounds have been shown to be bactericidal against pathogens [46], and to suppress the biological activity of toxins, including the cholera and ricin toxins [46,47,48,49]. Peas showed to bind with high efficiency Stx, acting as toxic-scavengers, whereas beans can reduce the intake of Stx [50].
2. Interactions between Phages and Bacteria in the Gut Microbiome
Phages were first described by the French-Canadian Félix d’Hérelle, of Institute Pasteur in 1917, who also defined the term ‘bacteriophage’ (“eater of bacteria”). As a first, pioneering phage-based therapy, he applied bacteriophages to treat Shigella infections in soldiers, establishing what became known as phage therapy [51,52,53]. Phages can be subdivided into two groups: virulent (lytic) and temperate (lysogenic) (Figure 1) [54]. Lytic viruses start the replication process soon after the infection of the bacterial host. Once the progeny virions have assembled in a sufficient number (the burst size), the cell bursts open, releasing the new phages in the surrounding environment. Lysogenic phages have an additional phase: they can integrate as prophages in the bacterial chromosome and undergo a latency period where only a viral transcription suppressor is produced actively. In particular contexts, such as bacterial starvation or DNA damage, the suppression control is relieved, and the prophage enters the lytic phase. Conversely, in the presence of a high number of infected bacteria, phages exit the lytic phase and initiate lysogeny [55]. Both virulent and temperate phages modulate the bacterial population through lysis.
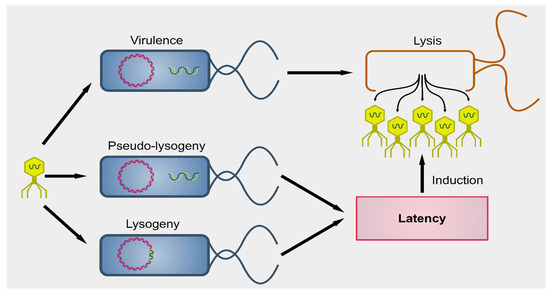
Figure 1. Outcomes of phagial infection of bacteria. A virulent phage (yellow particle on the left) can infect a bacterium (in blue). The replication of the phage leads to lysis of the host cell, releasing the viral progeny (yellow particles on the right). Alternatively, some viral species known as temperate can establish an additional step known as latency. The phagial genome can remain independent from that of the bacterium (pseudo-lysogeny) or become integrated into the host’s genome (lysogeny). In both cases, the viral expression is kept to a minimum and there is no virion production until several cellular conditions are met. Upon induction, temperate phages enter the lytic pathway and determine the lysis of the host.
Phages can also modulate the bacterial population, indirectly. It is well known that bacteria must undergo a fierce competition within each ecological niche, and, therefore, some species have developed virulence factors to improve their chances of survival [56]. Moreover, the microbial competition is complex and difficult to predict. For instance, Lactobacillus delbrueckii and L. rhamnosus inhibit E. coli O157, but L. plantarum suppresses the commensal strains of E. coli but not O157, and L. paracasei does not constrain E. coli at all [57]. In addition, the suppression of one species might cause the unexpected expansion of a species not apparently associated with the suppressed one. For instance, E. coli fosters the growth of B. fragilis but represses B. vulgatus. Knocking down E. coli by phage T4 is, therefore, followed by a contraction of the prevalence of B. fragilis and an increased growth of B. vulgatus, but also of Proteus mirabilis and Akkermansia muciniphila [58]. It is also known that commensal species can neutralize toxins, reducing the fitness of the pathogens. For instance, surface proteins of L. plantarum can neutralize Stx, reducing the cytotoxicity (and, thus, the fitness) of E. coli O157 [59]. Therefore, the alteration of even one species due to phagial predation can have drastic consequences for the microbiome.
Mounting evidence suggests that phages have access to eukaryotic (and human) cells [60]. Even though tissues are expected to be sterile, it has been known for decades that an ingestion of phage preparations during phage therapy is followed by a recovery of phages in human urine and blood within a few minutes from the administration [61,62]. This recovery implies that the viruses had somehow crossed the gastrointestinal barrier. Recent virome studies have identified genes belonging to phages in both blood and brain [63,64]. The circulation of phages in the peripheral blood has been named ‘phagemia’, but there is a lack of hard evidence for its actual existence in physiological conditions [65]. Furthermore, phages can be actively transported from one side to another of intestinal cells (transcytosis) via the Golgi network [16].
3. Effect of Dietary Compounds on Phages
Several dietary compounds can alter the physiology of phages, as summarized in Figure 2. Although many studies showed a connection between nutrition and intestinal microbiome, there are only a few studies that deal with the effects of nutrition on the activity of phages. Seminal work in the 1960 s indicated that amino acids and vitamins had a different impact on the induction of prophage λ in E. coli [66]. For instance, the amino acid cysteine was an inducer, but its oxidized derivative cystine was not. About four decades later, it was shown that essential oils extracted from chamomile, lemongrass, cinnamon, and geranium could greatly reduce the infectivity of E. coli T7 and S. aureus SA, whereas others (such as angelica, cardamom, lime, and rosemary) affected only the former phage [67]. A recent study reported how different compounds could selectively activate some viruses but not others in bacterial growth and prophage-induction assays [68]. This study demonstrated how stevia, a natural sweetener obtained from the Brazilian shrub Stevia rebaudiana [69], could strongly induce prophages present in Bacteroides thetaiotamicron and Staphylococcus aureus but not in Enterococcus faecalis, whereas uva ursi (derived from the shrub plant Arctostaphylos uva-ursi), aspartame (a peptide), and propolis (a flavonoid) resulted in the opposite. These data indicate that dietary compounds can modulate the gut virome and, consequently, alter the gut bacteriome.
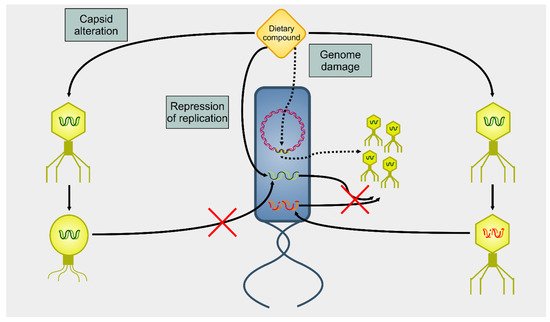
Figure 2. Summary of actions on phages on dietary compounds. There are three main mechanisms of action of dietary compounds upon phages. A dietary compound can modify the capsid, blocking the infectivity of the targeted phage (capsid alteration). Alternatively, dietary compounds can lead to the degradation of nucleic acids (genome damage). In this case, a phage can infect the host, but there will be neither lysis nor viral progeny. However, DNA damage to the host cell’s genome triggers the induction of prophages (dotted arrows). A final mechanism of action (repression of replication) involves an interference with the replication of the viral genome. Even in this case, there is infection, but no viral progeny is produced.
Experiments measuring the effect of dietary compounds on phage activity have been based on few classes of compounds, mainly polyphenols. These are molecules that contain one or more phenolic aromatic rings (benzenes with hydroxide moieties). Polyphenols can be subdivided into phenolic acid derivatives and flavonoids [70]. The former can, in turn, be subdivided into derivatives of either hydroxybenzoic acid (for instance, gallic acid) or cinnamic acid (for example, caffeic acid) [71]. Tea, the second most frequently consumed beverage after water, is a primary source for gallic acid [72]. Coffee, whose consumption is increasing worldwide [73], contains chlorogenic acid (a combination of caffeic acid and quinic acid) [71]. Tannic acid, which contains several hydroxybenzoic acid moieties, is particularly abundant in berries; soy is rich in isoflavonoids, such as genistein and daidzein [74]. The exact mechanism of action of these phenol-compounds is not entirely understood. Still, it is known that they can be beneficial for human physiology and have been used in folk medicine since millennia [75]. They are currently being investigated for their anticancer activity [76,77,78].
The chemical structure of the compounds discussed herein is shown in Figure 3, Figure 4 and Figure 5. A summary of the activities identified is given in Table 1. The most common outcome of exposure to a given nutrient is a loss of infectivity; this is measured by comparing the plaque-forming units (PFU) of a control and an exposed suspension (measured in mL) of phages. If the control and the exposed suspensions showed, for instance, 1010 and 109 PFU/mL, then the reduction is said to be one log10. Herein, we will report the results using this notation.
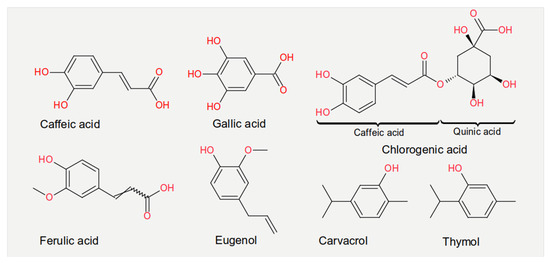
Figure 3. Chemical structures of the phenolic acids reported in the present review.
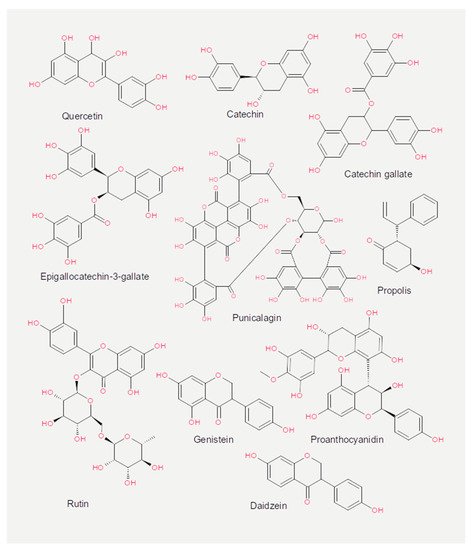
Figure 4. Chemical structures of the flavonoids reported in the present review.
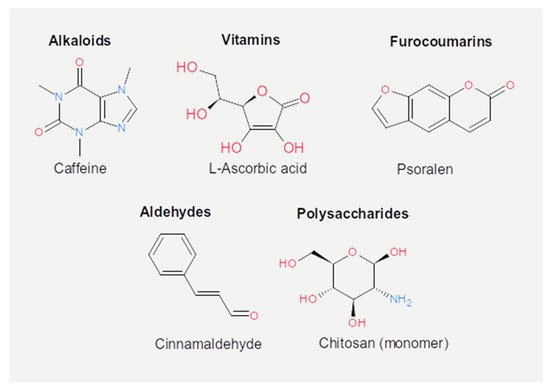
Figure 5. Chemical structures of the other active dietary compounds reported in the present review.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13092036
