
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lee Ann Applegate | + 10576 word(s) | 10576 | 2022-01-29 02:18:25 | | | |
| 2 | Alexis Laurent | + 2 word(s) | 10578 | 2022-01-29 08:55:21 | | | | |
| 3 | Amina Yu | -311 word(s) | 10267 | 2022-02-08 08:43:38 | | | | |
| 4 | Amina Yu | -9143 word(s) | 1124 | 2022-04-13 10:22:43 | | |
Video Upload Options
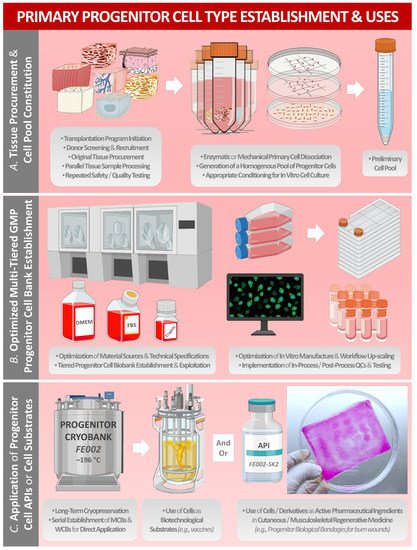
Historically, primary human progenitor cells (e.g., WI-38 and MRC-5 diploid-cell sources) have been industrially applied in research and in manufacturing processes for vaccines and for biologicals. Furthermore, tissue-specific primary progenitor-cell banks have recently been developed and exploited for the provision of safe, consistent, and effective cellular active pharmaceutical ingredients (API) in homologous allogeneic regenerative medicine applications. Notably, the modern legal and regulatory frameworks for novel therapeutic products and for progenitor-cell therapy development have been iteratively optimized to guarantee utmost product safety, quality, and efficacy. Over 50 years of global technical hindsight around progenitor-cell biotechnological substrates and over 30 years of in-house clinical experience around the therapeutic uses of standardized progenitor-cell sources in Switzerland have demonstrated the importance of such biological materials for public health. The aim of this entry work was to summarize the evolution of the industrial applications of selected primary progenitor-cell sources, ranging from the use as robust biotechnological substrates to standardized cellular API manufacture and their clinical uses in highly specialized regenerative medicine.
References
- Laurent, A.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Holistic approach of Swiss fetal progenitor cell banking: Optimizing safe and sustainable substrates for regenerative medicine and biotechnology. Front. Bioeng. Biotechnol. 2020, 8, 557758.
- Applegate, L.A.; Weber, D.; Simon, J.P.; Scaletta, C.; Hirt-Burri, N.; de Buys Roessingh, A.S.; Raffoul, W. Organ donation and whole-cell bioprocessing in the Swiss fetal progenitor cell transplantation platform. In Organ Donation and Organ Donors; Saidi, R.F., Ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 125–147. ISBN 978-1-62618-853-2.
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621.
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636.
- Jacobs, J.P.; Jones, C.M.; Baille, J.P. Characteristics of a human diploid cell designated MRC-5. Nature 1970, 227, 168–170.
- Laurent, A.; Abdel-Sayed, P.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A. Evolution of diploid progenitor lung cell applications: From optimized biotechnological substrates to potential active pharmaceutical ingredients in respiratory tract regenerative medicine. Cells 2021, 10, 2526.
- Olshansky, S.J.; Hayflick, L. The role of the WI-38 cell strain in saving lives and reducing morbidity. AIMS Public Health 2017, 4, 127–138.
- Hayflick, L. A novel technique for transforming the theft of mortal human cells into praiseworthy federal policy. Exp. Gerontol. 1998, 33, 191–207.
- Furton, E.J. Vaccines originating in abortion. Ethics Med. 1999, 24, 3–4.
- Maher, D.P.; Panicola, M.R.; Harte, C. Vaccines, abortions and moral coherence. Nat. Cathol. Bioethics Q. 2002, 2, 51–67.
- Rudd, G. Is vaccination complicit with abortion? Ann. Pharmacother. 2003, 37, 1340–1341.
- Ehreth, J. The global value of vaccination. Vaccine 2003, 21, 596–600.
- Pruss, A.R. Cooperation with past evil and use of cell-lines derived from aborted fetuses. Linacre Q. 2004, 71, 335–350.
- Leiva, R. Moral reflections on vaccines prepared from cells derived from aborted human fetuses. Nat. Cathol. Bioethics Q. 2006, 6, 541.
- Rodriguez Luno, A. Ethical reflections on vaccines using cells from aborted fetuses. Nat. Cathol. Bioethics Q. 2006, 6, 453–459.
- Norrby, E.; Prusiner, S.B. Polio and Nobel prizes: Looking back 50 years. Ann. Neurol. 2007, 61, 385–395.
- Ma, B.; He, L.F.; Zhang, Y.L.; Chen, M.; Wang, L.L.; Yang, H.W.; Yan, T.; Sun, M.X.; Zheng, C.Y. Characteristics and viral propagation properties of a new human diploid cell line, Walvax-2, and its suitability as a candidate cell substrate for vaccine production. Hum. Vaccines Immunother. 2015, 11, 998–1009.
- WHO Expert Committee on Biological Standardization. Recommendations for the Evaluation of Animal Cell Cultures as Substrates for the Manufacture of Biological Medicinal Products and for the Characterization of Cell Banks. Sixty-First Report, Annex 3; WHO Technical Report Series No. 978; World Health Organization: Geneva, Switzerland, 2013.
- Hawkins, R.; Stylianou, M. Expert Committee on Biological Standardization, Second replacement seed stock for MRC-5 cells. In Proposal for WHO Reference Cell Bank Status; WHO/BS/2018.2347; World Health Organization: Geneva, Switzerland, 2018.
- Laurent, A.; Lin, P.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; She, B.R.; Applegate, L.A. Bringing safe and standardized cell therapies to industrialized processing for burns and wounds. Front. Bioeng. Biotechnol. 2020, 8, 581.
- Vetro, S.W.; Bellanti, J.A. Fetal and neonatal immunoincompetence. Fetal Diagn. Ther. 1989, 4, 82–91.
- Abbaspanah, B.; Momeni, M.; Ebrahimi, M.; Mousavi, S.H. Advances in perinatal stem cells research: A precious cell source for clinical applications. Regen. Med. 2018, 13, 595–610.
- Bhattacharya, N. Fetal cell/tissue therapy in adult disease: A new horizon in regenerative medicine. Clin. Exp. Obstet. Gynecol. 2004, 31, 167–173.
- Clarkson, E.D. Fetal tissue transplantation for patients with Parkinson’s disease: A database of published clinical results. Drugs Aging 2001, 18, 773–785.
- Gaggi, G.; Izzicupo, P.; Di Credico, A.; Sancilio, S.; Di Baldassarre, A.; Ghinassi, B. Spare parts from discarded materials: Fetal annexes in regenerative medicine. Int. J. Mol. Sci. 2019, 20, 1573.
- Kaviani, A.; Guleserian, K.; Perry, T.E.; Jennings, R.W.; Ziegler, M.M.; Fauza, D.O. Fetal tissue engineering from amniotic fluid. J. Am. Coll. Surg. 2003, 196, 592–597.
- Kaviani, A.; Perry, T.E.; Barnes, C.M.; Oh, J.T.; Ziegler, M.M.; Fishman, S.J.; Fauza, D.O. The placenta as a cell source in fetal tissue engineering. J. Pediatr. Surg. 2002, 37, 995–999.
- Laurent, A.; Scaletta, C.; Hirt-Burri, N.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Swiss fetal transplantation program and nonenzymatically isolated primary progenitor cell types for regenerative medicine. In Stem Cells and Good Manufacturing Practices: Methods and Protocols; Kursad, T., Ed.; Springer Science+Business Media: New York, NY, USA, 2020.
- Beskow, L.M. Lessons from HeLa cells: The ethics and policy of biospecimens. Annu. Rev. Genom. Hum. Genet. 2016, 17, 395–417.
- Johnson, P.C.; Bertram, T.A.; Tawil, B.; Hellman, K.B. Hurdles in tissue engineering/regenerative medicine product commercialization: A survey of North American academia and industry. Tissue Eng. Part A 2011, 17, 5–15.
- Bertram, T.A.; Tentoff, E.; Johnson, P.C.; Tawil, B.; Van Dyke, M.; Hellman, K.B. Hurdles in tissue engineering/regenerative medicine product commercialization: A pilot survey of governmental funding agencies and the financial industry. Tissue Eng. Part A 2012, 18, 2187–2194.
- Pirnay, J.P.; Vanderkelen, A.; De Vos, D.; Draye, J.P.; Rose, T.; Ceulemans, C.; Ectors, N.; Huys, I.; Jennes, S.; Verbeken, G. Business oriented EU human cell and tissue product legislation will adversely impact Member States’ health care systems. Cell. Tissue Bank. 2013, 14, 525–560.
- Pearce, K.F.; Hildebrandt, M.; Greinix, H.; Scheding, S.; Koehl, U.; Worel, N.; Apperley, J.; Edinger, M.; Hauser, A.; Mischak-Weissinger, E.; et al. Regulation of advanced therapy medicinal products in Europe and the role of academia. Cytotherapy 2014, 16, 289–297.
- Ramezankhani, R.; Torabi, S.; Minaei, N.; Madani, H.; Rezaeiani, S.; Hassani, S.N.; Gee, A.P.; Dominici, M.; Silva, D.N.; Baharvand, H.; et al. Two decades of global progress in authorized advanced therapy medicinal products: An emerging revolution in therapeutic strategies. Front. Cell Dev. Biol. 2020, 8, 547653.
- Dimitropoulos, G.; Jafari, P.; de Buys Roessingh, A.; Hirt-Burri, N.; Raffoul, W.; Applegate, L.A. Burn patient care lost in good manufacturing practices? Ann. Burn. Fire Disasters 2016, 29, 111–115.
- Hartmann-Fritsch, F.; Marino, D.; Reichmann, E. About ATMPs, SOPs and GMP: The hurdles to produce novel skin grafts for clinical use. Transfus. Med. Hemother. 2016, 43, 344–352.
- De Wilde, S.; Veltrop-Duits, L.; Hoozemans-Strik, M.; Ras, T.; Blom-Veenman, J.; Guchelaar, H.J.; Zandvliet, M.; Meij, P. Hurdles in clinical implementation of academic advanced therapy medicinal products: A national evaluation. Cytotherapy 2016, 18, 797–805.
- Hohlfeld, J.; de Buys Roessingh, A.S.; Hirt-Burri, N.; Chaubert, P.; Gerber, S.; Scaletta, C.; Hohlfeld, P.; Applegate, L.A. Tissue engineered fetal skin constructs for pediatric burns. Lancet 2005, 366, 840–842.
- Hirt-Burri, N.; Ramelet, A.A.; Raffoul, W.; de Buys Roessingh, A.S.; Scaletta, C.; Pioletti, D.P.; Applegate, L.A. Biologicals and fetal cell therapy for wound and scar management. ISRN Dermatol. 2011, 2011, 549870.
- De Buys Roessingh, A.S.; Hirt-Burri, N.; Raffoul, W.; Scaletta, C.; Applegate, L.A. A decade after foetal skin progenitor cell therapy in pediatric burn treatment. J. Regen. Med. 2015, 4, 1.
- Al-Dourobi, K.; Laurent, A.; Deghayli, L.; Flahaut, M.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; Waselle, L.; Simon, J.P.; Ezzi, O.E.; et al. Retrospective evaluation of progenitor biological bandage use: A complementary and safe therapeutic management option for prevention of hypertrophic scarring in pediatric burn care. Pharmaceuticals 2021, 14, 201.
- Abdel-Sayed, P.; Hirt-Burri, N.; de Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Evolution of biological bandages as first cover for burn patients. Adv. Wound Care 2019, 8, 555–564.
- Ramelet, A.A.; Hirt-Burri, N.; Raffoul, W.; Scaletta, C.; Pioletti, D.P.; Offord, E.; Mansourian, R.; Applegate, L.A. Chronic wound healing by fetal cell therapy may be explained by differential gene profiling observed in fetal versus old skin cells. Exp. Gerontol. 2009, 44, 208–218.
- Quintin, A.; Hirt-Burri, N.; Scaletta, C.; Schizas, C.; Pioletti, D.P.; Applegate, L.A. Consistency and safety of cell banks for research and clinical use: Preliminary analysis of fetal skin banks. Cell Transplant. 2007, 16, 675–684.
- Darwiche, S.E.; Scaletta, C.; Raffoul, W.; Pioletti, D.P.; Applegate, L.A. Epiphyseal chondroprogenitors provide a stable cell source for cartilage cell therapy. Cell Med. 2012, 4, 23–32.
- Grognuz, A.; Scaletta, C.; Farron, A.; Raffoul, W.; Applegate, L.A. Human fetal progenitor tenocytes for regenerative medicine. Cell Transplant. 2016, 25, 463–479.
- Laurent, A.; Darwiche, S.E.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; Laurent, P.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Banking progenitor cells for hippiatric regenerative medicine: Optimized establishment of safe and consistent cell sources for standardized veterinary therapeutic protocols. AJBSR 2020, 8, 252–271.
- Hirt-Burri, N.; de Buys Roessingh, A.S.; Scaletta, C.; Gerber, S.; Pioletti, D.P.; Applegate, L.A.; Hohlfeld, J. Human muscular fetal cells: A potential cell source for muscular therapies. Pediatr. Surg. Int. 2008, 24, 37–47.
- Quintin, A.; Schizas, C.; Scaletta, C.; Jaccoud, S.; Gerber, S.; Osterheld, M.C.; Jullierat, L.; Applegate, L.A.; Pioletti, D.P. Isolation and in vitro chondrogenic potential of human foetal spine cells. J. Cell Mol. Med. 2009, 13, 2559–2569.
- Montjovent, M.O.; Burri, N.; Mark, S.; Federici, E.; Scaletta, C.; Zambelli, P.Y.; Hohlfeld, P.; Leyvraz, P.F.; Applegate, L.A.; Pioletti, D.P. Fetal bone cells for tissue engineering. Bone 2004, 35, 1323–1333.
- Laurent, A.; Scaletta, C.; Abdel-Sayed, P.; Michetti, M.; Flahaut, M.; Simon, J.P.; de Buys Roessingh, A.S.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A. Optimized manufacture of lyophilized dermal fibroblasts for next-generation off-the-shelf progenitor biological bandages in topical post-burn regenerative medicine. Biomedicines 2021, 9, 1072.
- Laurent, A.; Abdel-Sayed, P.; Ducrot, A.; Hirt-Burri, N.; Scaletta, C.; Jaccoud, S.; Nuss, K.; de Buys Roessingh, A.S.; Raffoul, W.; Pioletti, D.P.; et al. Development of standardized fetal progenitor cell therapy for cartilage regenerative medicine: Industrial transposition and preliminary safety in xenogeneic transplantation. Biomolecules 2021, 11, 250.
- Laurent, A.; Abdel-Sayed, P.; Grognuz, A.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Kronen, P.; Nuss, K.; et al. Industrial development of standardized fetal progenitor cell therapy for tendon regenerative medicine: Preliminary safety in xenogeneic transplantation. Biomedicines 2021, 9, 380.

