Historically, primary human progenitor cells (e.g., WI-38 and MRC-5 diploid-cell sources) have been industrially applied in research and in manufacturing processes for vaccines and for biologicals. Furthermore, tissue-specific primary progenitor-cell banks have recently been developed and exploited for the provision of safe, consistent, and effective cellular active pharmaceutical ingredients (API) in homologous allogeneic regenerative medicine applications. Notably, the modern legal and regulatory frameworks for novel therapeutic products and for progenitor-cell therapy development have been iteratively optimized to guarantee utmost product safety, quality, and efficacy. Over 50 years of global technical hindsight around progenitor-cell biotechnological substrates and over 30 years of in-house clinical experience around the therapeutic uses of standardized progenitor-cell sources in Switzerland have demonstrated the importance of such biological materials for public health. The aim of this entry work was to summarize the evolution of the industrial applications of selected primary progenitor-cell sources, ranging from the use as robust biotechnological substrates to standardized cellular API manufacture and their clinical uses in highly specialized regenerative medicine.
- biotechnological substrates
- cell therapies
- organ donation
- pharmacopeial monographs
- progen-itor cells
- quality requirements
- regenerative medicine
- regulatory compliance
- standardized transplants
- vaccine substrates
1. Introduction
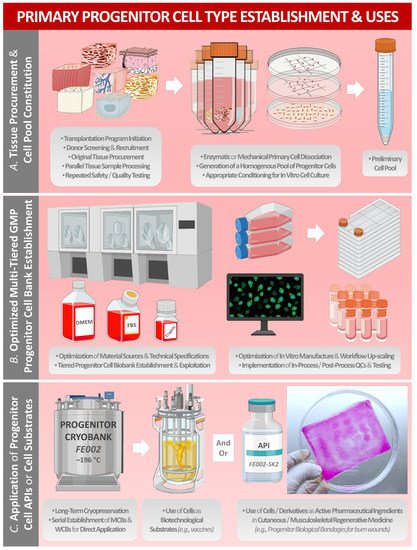
2. Methodological Aspects of Starting Biological Material Procurement for Modern Primary Progenitor-Cell-Type Establishment
| Personnel Identification |
Key Professional Competences | Roles in the Cell-Transplantation Program |
|---|
| Material Safety and Quality Assessments: Testing Class and Testing Parameters |
Considered Cell Bank Tiers and Applicability of Testing |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cell Seed/PCB | MCB | WCB | ECB/EOPCB | |||||
| Program Manager 1 | Experience in tissue and cell banking. Qualifications for GMP cell manufacture. Experience in multidisciplinary professional group coordination. |
Selection of the optimal biological starting materials. Establishment and coordination of the cell-transplantation program; acting as the legally responsible person. Custodian of the cell-transplantation-program records and biobank administrator. |
||||||
| 1. Identity and Purity | Cellular morphology | + | + | + | + | Technical Manager 1 | Experience in tissue and cell banking. Qualifications for GMP cell manufacture. |
Selection of the optimal biological starting materials. Oversight of the tissue processing, in vitro cell culture initiation, and of cell banking. Responsible person for GMP processes. |
| Medical Doctor | ||||||||
| Cell-type identification (genetic and biochemical, immunological, or cytogenetic profiling) | Experience in medical pregnancy termination. | Screening of potential donors, eligibility documentation, procurement of the donation, and implementation of the biospecimen and information coding. | ||||||
| Legal Advisor | Experience in applicable laws and regulations on transplantation and on therapeutic products. | Support in the establishment of the cell-transplantation program and liaison with local ethics committees and with local or national health authorities. | ||||||
| Pathologist | Experience in pre-natal pathology and in histopathology. | Anatomical and histopathological QC examination of the donated tissues. | ||||||
| Immunologist | Experience in medical analytics. Qualifications and accreditations for pathogen screening and sample analysis. |
Screening for pathogen detection in the blood samples of potential donors and QC testing of the donated materials and of the progeny cells. | ||||||
-
Donor of female sex and female gender.
-
Donor of biological age between 18 years and 25 years.
| + | ||||
| + | + | + | ||
| Cell-type karyotype (diploid cells) | + | + | + (1) | + (1) |
| Cell type in vitro lifespan (diploid cells) | − | + | + | − |
| 2. Extraneous Agents | Tests for bacterial and fungal contamination |
- Donation performed at 12–14 weeks following conception.
-
Donor of specified nationality and place of residence.
-
Donor pregnancy resulting from natural insemination.
-
Donor performing voluntary, gratuitous, and legal donation.
-
Documentation of informed consent for the gestation termination.
-
Documentation of informed consent for the donation.
-
Donor in good overall health.
-
Donor not suffering from any chronic disease or affection.
-
Qualifying serological screening of the donor for specified pathogens (e.g., HIV, HBV, HCV, HTLV, CMV, S-West Nile virus, Treponema pallidum, Toxoplasma gondii, etc.).
-
Availability of the donor after three months for repeat serological testing.
-
No treatment of the donor with anti-inflammatory or other therapeutic drugs in the past six months.
-
No inoculation of the donor with vaccines in the past four weeks.
-
No donor history of post-travel sickness in the past six months.
-
No history of immunotherapeutic treatment of the donor.
-
No history of transplantation receipt by the donor.
-
No history of Creutzfeldt–Jacob disease in the donor’s family.
3. Optimized Modern Industrial Manufacturing of Primary Progenitor Cells in Tiered Cell-Bank Systems
| Nomenclature Terms | Term Definitions/Material Descriptions | ||
|---|---|---|---|
| Cell Bank System | A system whereby the successive final lots of a product are manufactured by culture in cells derived from the same MCB 1. A number of containers from the MCB are used to prepare a WCB. The in vitro age of the cells is counted from the MCB 2. The cell-bank system is validated for the highest in vitro passage level achieved during routine production 3. The use, identity, and inventory control of the individual cell-bank containers is carefully documented. | ||
| Diploid Cell Type | Cryopreserved stocks and cultures of diploid cells that have a high but finite capacity for multiplication in vitro | ||
| − | + | + | − |
| Tests for mycobacteria |
4. Industrial Applications of Primary Progenitor Cells as Biotechnological Substrates or as Therapeutic APIs and Related Quality Requirements
| Target Diseases | Diploid Cell Substrate Types | Identified Industrial Manufacturers | ||||||
|---|---|---|---|---|---|---|---|---|
| Smallpox | MRC-5 | Acambis | 4. In diploid cell types, the cells have essentially the same characteristics as those of the tissues of origin. | |||||
| Chickenpox | WI-38, MRC-5 | Merck, GlaxoSmithKline | Primary Cell Cultures | Cultures of cells directly obtained by trypsinization or by mechanical treatment of a suitable starting tissue or organ fragment 5. The cells are essentially identical to those of the tissue of origin and are no more than five in vitro passages from the initial preparation from the mammalian tissue of origin. The primary cell cultures are harvested to form the preliminary cell pool. | ||||
| Shingles | WI-38, MRC-5 | Merck | Parental Cell Bank (PCB) | A preliminary cell pool distributed into containers in a single operation, processed together, and stored in such a manner as to ensure uniformity and stability and to prevent contamination 6. | ||||
| Poliomyelitis | MRC-5 | Sanofi Pasteur | Master Cell Bank (MCB) | A culture of cells derived from the cell seed/PCB, distributed into containers in a single operation, processed together, and stored in such a manner as to ensure uniformity and stability and to prevent contamination. The MCBs are usually stored at −70 °C or at lower temperatures 7. | − | + (2) | + (2) | − |
| Tests for mycoplasmas | − | + | + | − | ||||
| Tests for spiroplasmas (3) | − | + | + | − | ||||
| Electron microscopy | − | + (4) | − | + (4) | ||||
| Tests for extraneous agents in cell cultures (intact cells or equivalent cell lysates) | − | + | + | + | ||||
| Tests in animals and in eggs | − | − | + (5) | + (5) | ||||
| Tests for specific viruses | − | + (6) | + (6) | + (6) | ||||
| Tests for retroviruses | − | + (4) | − | + (4) | ||||
| 3. Tumorigenicity | Cell-type tumorigenicity | + (7,8) | − | − | + (7) |

| Mumps | ||||
| WI-38 | Merck | |||
| Working Cell Bank (WCB) | A culture of cells derived from a MCB and intended for use in the preparation of production cell cultures. The WCB lot is distributed into containers, processed, and is stored as described for the MCBs. | |||
| Measles | WI-38 | Merck, GlaxoSmithKline | Production Cell Culture | A culture of cells intended for use in production; it may be derived from one or more containers of a WCB or it may be a primary cell culture. |
| End-of-Production Cell Bank (EOPCB) or Extended Cell Bank (ECB) | A culture of cells derived from a WCB, at or beyond the maximum in vitro cell-population-doubling level used for production, distributed into containers in a single operation, processed together, and stored in such a manner as to ensure uniformity and stability and to prevent contamination 3. | |||
| Final Lot or Batch | A collection of closed, final containers or other final dosage units that are expected to be homogenous and equivalent with respect to risk of contamination during filling or preparation of the final product. The dosage units are filled, or otherwise prepared, from the same final bulk and are closed in one continuous working session. They bare a distinctive number or code identifying the final lot or batch. |
| Rubella | ||
| WI-38 | ||
| Merck | ||
| Hepatitis A | MRC-5 | Merck, GlaxoSmithKline, Sanofi, Berna |
| Hepatitis B | MRC-5 | GlaxoSmithKline, Sanofi |
| Typhoid | MRC-5 | Sanofi Pasteur |
| Rabies | MRC-5 | Sanofi Pasteur |
-
Full traceability may be made available back to the (anonymized) origin of the primary cell source, around the derivation of the primary cell type and around the materials used in the preparation of the cell seed stocks.
-
The technical possibility exists to create, by means of serial in vitro sub-cultures in a defined sustainable cell-bank system, sufficient amounts of consistent populations of cells to satisfy a globalized industrial-scale demand for high-quality biological substrates (e.g., millions of standardized WCB vials of the same original cell type).
-
The technical possibility exists to cryopreserve extensive and homogenous cell lots at relatively early population-doubling levels or passage levels within the qualified in vitro lifespan of the primary cell type of interest.
-
A standardized approach may be adopted for the original establishment of appropriate cell types and for the characterization of the derived cryopreserved cell banks, which may later be used as starting materials for further multi-tiered cell banking and for the sustainable provision of standardized cell sources for research and industrial applications.
-
The technical possibility exists (i.e., due to the high sustainability of the cell sources) for the implementation of extensive and appropriate (i.e., risk analysis-based) biosafety testing schemes for the qualification of manufacturer-specific cell banks before the use in vaccine-production activities.
-
Numerous technical possibilities exist for the demonstration that the considered cellular materials are exempt from detectable adventitious agents and that they are unable to form tumors when inoculated into immunosuppressed animal models or equivalent models.
-
The possibility exists that the original research is subject to open international scientific scrutiny and to collaborative technical investigations of the characteristics of the cells or of the possible presence of adventitious agents. The cell-characterization results may be peer-reviewed and published.
-
A single source of cells may exist, with a growing scientifically and technically updated body of safety-testing data and a safe history of use, giving increased confidence to manufacturers, regulators, and public-policy makers.
-
The supply of cells may be free of any constraint related to intellectual-property rights on final products.
-
The possibility exists, based on intrinsic cellular characteristics, to propagate diverse viral materials (i.e., viral pathogens infecting humans) with extremely high efficiency of replication in the manufactured primary cell populations.
- (i)
-
Product and process development stages, in order to identify the optimal mechanisms for viral-material replication and to select the optimal manufacturing processes and materials to be used for production.
- (ii)
-
Process confirmation or validation stages, in order to ensure that the selected processes and materials may be tangibly transposed into production.
- (iii)
-
Production-validation stages, in order to validate that the selected substrates, materials, and processes may be tangibly used in the final manufacturing system for the final-product formula.
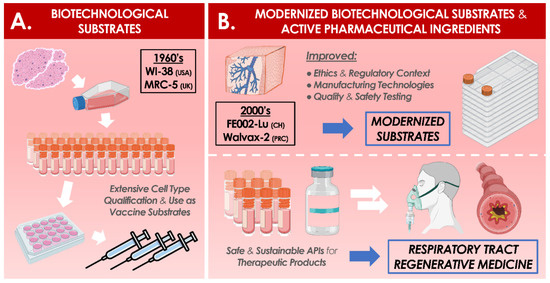
5. Formulation Possibilities for Therapeutic Primary Progenitor Cells in Tissue Engineering and in Regenerative Medicine

6. Regulatory Considerations for Therapeutic Primary Progenitor Cells and for Related Regenerative Medicine Products
| Product Category (Jurisdiction) |
Therapeutic-Product Definition Excerpts 1 |
|---|---|
| ATMP (EU) |
A medicinal product for human use consisting of (…), a somatic cell therapy medicinal product, or a tissue-engineered product. Somatic-cell-therapy medicinal product means a biological medicinal product that contains or consists of cells or tissues that have been subject to substantial manipulation so that the biological characteristics, physiological functions, or structural properties relevant for the intended clinical use have been altered, or of cells or tissues that are not intended to be used for the same essential function(s) in the recipient and the donor and is presented as having properties for, or is used in or administered to human beings with a view to treating, preventing, or diagnosing a disease through the pharmacological, immunological, or metabolic action of its cells or tissues. |
| cATMP (EU) |
An ATMP that must incorporate, as an integral part of the product, one or more medical devices (…), and its cellular or tissue part must contain viable cells or tissues, or its cellular or tissue part containing non-viable cells or tissues must be liable to act upon the human body with an action that can be considered as primary to that of the devices (…). |
| TEP (EU) |
A product that contains or consists of engineered cells or tissues, and is presented as having properties for, or is used in or administered to human beings with a view to regenerating, repairing, or replacing a human tissue. A tissue-engineered product may contain cells or tissues of human or animal origin, or both. The cells or tissues may be viable or non-viable. It may also contain additional substances, such as cellular products, bio-molecules, biomaterials, chemical substances, scaffolds, or matrices. Products containing or consisting exclusively of non-viable human or animal cells and/or tissues, which do not contain any viable cells or tissues and which do not act principally by pharmacological, immunological, or metabolic action, shall be excluded from this definition. |
| TrSt (CH) |
A transplant product that is intended for transfer to a human and/or whose production process can be standardized, which consists of, or contains, autogenous, allogeneic, or xenogeneic vital organs, tissues, or cells and which is manufactured by means of a standardized procedure. When transplanted, these organs, tissues, or cells are generally manipulated in such a way that their original biological characteristics, physiological functions, or structural properties are affected, or the cells or tissues are not intended to perform essentially the same function(s) in the recipient as in the donor. These can be products from somatic-cell therapy (…) or tissue engineering. Among other aspects, the transplant product serves to regenerate, improve, or influence the human physiological body functions by means of a pharmacological, immunological, or metabolic effect on humans, or it can be used to replace human tissue in order to heal or protect against illnesses, injuries, or impairments. |
7. Preclinical and Clinical Experience with Therapeutic Primary Progenitor Cells in the Swiss Progenitor-Cell-Transplantation Program
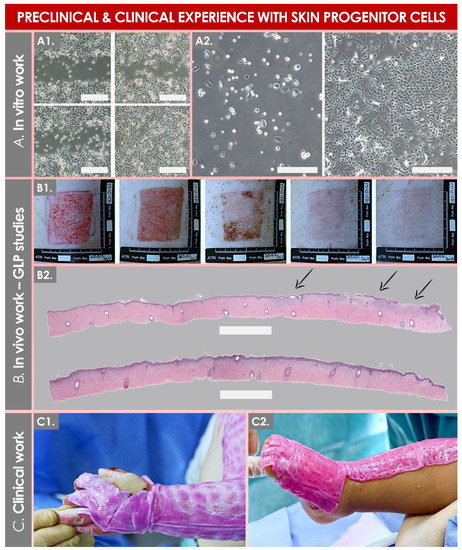
| Starting Tissue Types |
Progenitor-Cell-Type Examples | Research, Preclinical, and Clinical Application Work and Milestones |
Cell-Type Deposit References |
|---|---|---|---|
| Skin | FE002-SK2 1 | Manufacturing: Industrial GMP cell-manufacturing upscaling and international transposition [20][51]. Clinical trials: Severe burns, refractory cutaneous ulcers, and skin donor-site wounds [38][40][41][43]. |
ECACC 12070301-FE002-SK2; FIRDI BCRC 960460 |
| Cartilage | FE002-Cart 1 | Manufacturing: Industrial cell-banking and cell-manufacturing optimization [52]. Preclinical studies: Safety of cell transplantation in caprine model [52]. |
ECACC 12070303-FE002-Cart; FIRDI BCRC 960459 |
| Tendon | FE002-Ten 1 | Manufacturing: Industrial cell-banking and cell-manufacturing optimization [53][94]. Preclinical studies: Safety of cell transplantation in lagomorph model [53]. |
ECACC 12070302-FE002-Ten; FIRDI BCRC 960461 |
| Bone | FE002-Bone | Manufacturing: Optimized cell banking and cell manufacturing [50]. Preclinical studies: Safety of cell transplantation in rat models [103]. |
NA |
| Muscle | FE002-Mu | Manufacturing: Optimized cell banking and cell manufacturing [48]. Preclinical studies: Safety of cell transplantation in murine model [104]. |
NA |
| Intervertebral disc | FE002-Disc | Manufacturing: Optimized cell banking and cell manufacturing. | NA |
| Lung | FE002-Lu | Manufacturing: Optimized cell banking and cell manufacturing. | NA |
8. Conclusions
References
- Laurent, A.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Holistic approach of Swiss fetal progenitor cell banking: Optimizing safe and sustainable substrates for regenerative medicine and biotechnology. Front. Bioeng. Biotechnol. 2020, 8, 557758.
- Applegate, L.A.; Weber, D.; Simon, J.P.; Scaletta, C.; Hirt-Burri, N.; de Buys Roessingh, A.S.; Raffoul, W. Organ donation and whole-cell bioprocessing in the Swiss fetal progenitor cell transplantation platform. In Organ Donation and Organ Donors; Saidi, R.F., Ed.; Nova Science Publishers: New York, NY, USA, 2013; pp. 125–147. ISBN 978-1-62618-853-2.
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621.
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636.
- Jacobs, J.P.; Jones, C.M.; Baille, J.P. Characteristics of a human diploid cell designated MRC-5. Nature 1970, 227, 168–170.
- Laurent, A.; Abdel-Sayed, P.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A. Evolution of diploid progenitor lung cell applications: From optimized biotechnological substrates to potential active pharmaceutical ingredients in respiratory tract regenerative medicine. Cells 2021, 10, 2526.
- Olshansky, S.J.; Hayflick, L. The role of the WI-38 cell strain in saving lives and reducing morbidity. AIMS Public Health 2017, 4, 127–138.
- Hayflick, L. A novel technique for transforming the theft of mortal human cells into praiseworthy federal policy. Exp. Gerontol. 1998, 33, 191–207.
- Furton, E.J. Vaccines originating in abortion. Ethics Med. 1999, 24, 3–4.
- Maher, D.P.; Panicola, M.R.; Harte, C. Vaccines, abortions and moral coherence. Nat. Cathol. Bioethics Q. 2002, 2, 51–67.
- Rudd, G. Is vaccination complicit with abortion? Ann. Pharmacother. 2003, 37, 1340–1341.
- Ehreth, J. The global value of vaccination. Vaccine 2003, 21, 596–600.
- Pruss, A.R. Cooperation with past evil and use of cell-lines derived from aborted fetuses. Linacre Q. 2004, 71, 335–350.
- Leiva, R. Moral reflections on vaccines prepared from cells derived from aborted human fetuses. Nat. Cathol. Bioethics Q. 2006, 6, 541.
- Rodriguez Luno, A. Ethical reflections on vaccines using cells from aborted fetuses. Nat. Cathol. Bioethics Q. 2006, 6, 453–459.
- Norrby, E.; Prusiner, S.B. Polio and Nobel prizes: Looking back 50 years. Ann. Neurol. 2007, 61, 385–395.
- Ma, B.; He, L.F.; Zhang, Y.L.; Chen, M.; Wang, L.L.; Yang, H.W.; Yan, T.; Sun, M.X.; Zheng, C.Y. Characteristics and viral propagation properties of a new human diploid cell line, Walvax-2, and its suitability as a candidate cell substrate for vaccine production. Hum. Vaccines Immunother. 2015, 11, 998–1009.
- WHO Expert Committee on Biological Standardization. Recommendations for the Evaluation of Animal Cell Cultures as Substrates for the Manufacture of Biological Medicinal Products and for the Characterization of Cell Banks. Sixty-First Report, Annex 3; WHO Technical Report Series No. 978; World Health Organization: Geneva, Switzerland, 2013.
- Hawkins, R.; Stylianou, M. Expert Committee on Biological Standardization, Second replacement seed stock for MRC-5 cells. In Proposal for WHO Reference Cell Bank Status; WHO/BS/2018.2347; World Health Organization: Geneva, Switzerland, 2018.
- Laurent, A.; Lin, P.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; She, B.R.; Applegate, L.A. Bringing safe and standardized cell therapies to industrialized processing for burns and wounds. Front. Bioeng. Biotechnol. 2020, 8, 581.
- Vetro, S.W.; Bellanti, J.A. Fetal and neonatal immunoincompetence. Fetal Diagn. Ther. 1989, 4, 82–91.
- Abbaspanah, B.; Momeni, M.; Ebrahimi, M.; Mousavi, S.H. Advances in perinatal stem cells research: A precious cell source for clinical applications. Regen. Med. 2018, 13, 595–610.
- Bhattacharya, N. Fetal cell/tissue therapy in adult disease: A new horizon in regenerative medicine. Clin. Exp. Obstet. Gynecol. 2004, 31, 167–173.
- Clarkson, E.D. Fetal tissue transplantation for patients with Parkinson’s disease: A database of published clinical results. Drugs Aging 2001, 18, 773–785.
- Gaggi, G.; Izzicupo, P.; Di Credico, A.; Sancilio, S.; Di Baldassarre, A.; Ghinassi, B. Spare parts from discarded materials: Fetal annexes in regenerative medicine. Int. J. Mol. Sci. 2019, 20, 1573.
- Kaviani, A.; Guleserian, K.; Perry, T.E.; Jennings, R.W.; Ziegler, M.M.; Fauza, D.O. Fetal tissue engineering from amniotic fluid. J. Am. Coll. Surg. 2003, 196, 592–597.
- Kaviani, A.; Perry, T.E.; Barnes, C.M.; Oh, J.T.; Ziegler, M.M.; Fishman, S.J.; Fauza, D.O. The placenta as a cell source in fetal tissue engineering. J. Pediatr. Surg. 2002, 37, 995–999.
- Laurent, A.; Scaletta, C.; Hirt-Burri, N.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Swiss fetal transplantation program and nonenzymatically isolated primary progenitor cell types for regenerative medicine. In Stem Cells and Good Manufacturing Practices: Methods and Protocols; Kursad, T., Ed.; Springer Science+Business Media: New York, NY, USA, 2020.
- Beskow, L.M. Lessons from HeLa cells: The ethics and policy of biospecimens. Annu. Rev. Genom. Hum. Genet. 2016, 17, 395–417.
- Johnson, P.C.; Bertram, T.A.; Tawil, B.; Hellman, K.B. Hurdles in tissue engineering/regenerative medicine product commercialization: A survey of North American academia and industry. Tissue Eng. Part A 2011, 17, 5–15.
- Bertram, T.A.; Tentoff, E.; Johnson, P.C.; Tawil, B.; Van Dyke, M.; Hellman, K.B. Hurdles in tissue engineering/regenerative medicine product commercialization: A pilot survey of governmental funding agencies and the financial industry. Tissue Eng. Part A 2012, 18, 2187–2194.
- Pirnay, J.P.; Vanderkelen, A.; De Vos, D.; Draye, J.P.; Rose, T.; Ceulemans, C.; Ectors, N.; Huys, I.; Jennes, S.; Verbeken, G. Business oriented EU human cell and tissue product legislation will adversely impact Member States’ health care systems. Cell. Tissue Bank. 2013, 14, 525–560.
- Pearce, K.F.; Hildebrandt, M.; Greinix, H.; Scheding, S.; Koehl, U.; Worel, N.; Apperley, J.; Edinger, M.; Hauser, A.; Mischak-Weissinger, E.; et al. Regulation of advanced therapy medicinal products in Europe and the role of academia. Cytotherapy 2014, 16, 289–297.
- Ramezankhani, R.; Torabi, S.; Minaei, N.; Madani, H.; Rezaeiani, S.; Hassani, S.N.; Gee, A.P.; Dominici, M.; Silva, D.N.; Baharvand, H.; et al. Two decades of global progress in authorized advanced therapy medicinal products: An emerging revolution in therapeutic strategies. Front. Cell Dev. Biol. 2020, 8, 547653.
- Dimitropoulos, G.; Jafari, P.; de Buys Roessingh, A.; Hirt-Burri, N.; Raffoul, W.; Applegate, L.A. Burn patient care lost in good manufacturing practices? Ann. Burn. Fire Disasters 2016, 29, 111–115.
- Hartmann-Fritsch, F.; Marino, D.; Reichmann, E. About ATMPs, SOPs and GMP: The hurdles to produce novel skin grafts for clinical use. Transfus. Med. Hemother. 2016, 43, 344–352.
- De Wilde, S.; Veltrop-Duits, L.; Hoozemans-Strik, M.; Ras, T.; Blom-Veenman, J.; Guchelaar, H.J.; Zandvliet, M.; Meij, P. Hurdles in clinical implementation of academic advanced therapy medicinal products: A national evaluation. Cytotherapy 2016, 18, 797–805.
- Hohlfeld, J.; de Buys Roessingh, A.S.; Hirt-Burri, N.; Chaubert, P.; Gerber, S.; Scaletta, C.; Hohlfeld, P.; Applegate, L.A. Tissue engineered fetal skin constructs for pediatric burns. Lancet 2005, 366, 840–842.
- Hirt-Burri, N.; Ramelet, A.A.; Raffoul, W.; de Buys Roessingh, A.S.; Scaletta, C.; Pioletti, D.P.; Applegate, L.A. Biologicals and fetal cell therapy for wound and scar management. ISRN Dermatol. 2011, 2011, 549870.
- De Buys Roessingh, A.S.; Hirt-Burri, N.; Raffoul, W.; Scaletta, C.; Applegate, L.A. A decade after foetal skin progenitor cell therapy in pediatric burn treatment. J. Regen. Med. 2015, 4, 1.
- Al-Dourobi, K.; Laurent, A.; Deghayli, L.; Flahaut, M.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; Waselle, L.; Simon, J.P.; Ezzi, O.E.; et al. Retrospective evaluation of progenitor biological bandage use: A complementary and safe therapeutic management option for prevention of hypertrophic scarring in pediatric burn care. Pharmaceuticals 2021, 14, 201.
- Abdel-Sayed, P.; Hirt-Burri, N.; de Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Evolution of biological bandages as first cover for burn patients. Adv. Wound Care 2019, 8, 555–564.
- Ramelet, A.A.; Hirt-Burri, N.; Raffoul, W.; Scaletta, C.; Pioletti, D.P.; Offord, E.; Mansourian, R.; Applegate, L.A. Chronic wound healing by fetal cell therapy may be explained by differential gene profiling observed in fetal versus old skin cells. Exp. Gerontol. 2009, 44, 208–218.
- Quintin, A.; Hirt-Burri, N.; Scaletta, C.; Schizas, C.; Pioletti, D.P.; Applegate, L.A. Consistency and safety of cell banks for research and clinical use: Preliminary analysis of fetal skin banks. Cell Transplant. 2007, 16, 675–684.
- Darwiche, S.E.; Scaletta, C.; Raffoul, W.; Pioletti, D.P.; Applegate, L.A. Epiphyseal chondroprogenitors provide a stable cell source for cartilage cell therapy. Cell Med. 2012, 4, 23–32.
- Grognuz, A.; Scaletta, C.; Farron, A.; Raffoul, W.; Applegate, L.A. Human fetal progenitor tenocytes for regenerative medicine. Cell Transplant. 2016, 25, 463–479.
- Laurent, A.; Darwiche, S.E.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; Laurent, P.; Raffoul, W.; de Buys Roessingh, A.S.; Applegate, L.A. Banking progenitor cells for hippiatric regenerative medicine: Optimized establishment of safe and consistent cell sources for standardized veterinary therapeutic protocols. AJBSR 2020, 8, 252–271.
- Hirt-Burri, N.; de Buys Roessingh, A.S.; Scaletta, C.; Gerber, S.; Pioletti, D.P.; Applegate, L.A.; Hohlfeld, J. Human muscular fetal cells: A potential cell source for muscular therapies. Pediatr. Surg. Int. 2008, 24, 37–47.
- Quintin, A.; Schizas, C.; Scaletta, C.; Jaccoud, S.; Gerber, S.; Osterheld, M.C.; Jullierat, L.; Applegate, L.A.; Pioletti, D.P. Isolation and in vitro chondrogenic potential of human foetal spine cells. J. Cell Mol. Med. 2009, 13, 2559–2569.
- Montjovent, M.O.; Burri, N.; Mark, S.; Federici, E.; Scaletta, C.; Zambelli, P.Y.; Hohlfeld, P.; Leyvraz, P.F.; Applegate, L.A.; Pioletti, D.P. Fetal bone cells for tissue engineering. Bone 2004, 35, 1323–1333.
- Laurent, A.; Scaletta, C.; Abdel-Sayed, P.; Michetti, M.; Flahaut, M.; Simon, J.P.; de Buys Roessingh, A.S.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A. Optimized manufacture of lyophilized dermal fibroblasts for next-generation off-the-shelf progenitor biological bandages in topical post-burn regenerative medicine. Biomedicines 2021, 9, 1072.
- Laurent, A.; Abdel-Sayed, P.; Ducrot, A.; Hirt-Burri, N.; Scaletta, C.; Jaccoud, S.; Nuss, K.; de Buys Roessingh, A.S.; Raffoul, W.; Pioletti, D.P.; et al. Development of standardized fetal progenitor cell therapy for cartilage regenerative medicine: Industrial transposition and preliminary safety in xenogeneic transplantation. Biomolecules 2021, 11, 250.
- Laurent, A.; Abdel-Sayed, P.; Grognuz, A.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Kronen, P.; Nuss, K.; et al. Industrial development of standardized fetal progenitor cell therapy for tendon regenerative medicine: Preliminary safety in xenogeneic transplantation. Biomedicines 2021, 9, 380.
- European Parliament and Council. Directive 2004/23/EC on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. Off. J. Eur. Union 2004, L 102, 48–58. Available online: http://data.europa.eu/eli/dir/2004/23/oj (accessed on 1 December 2021).
- European Commission. Commission Directive (EC) No 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. Off. J. Eur. Union 2006, L38, 40–52. Available online: http://data.europa.eu/eli/dir/2006/17/oj (accessed on 1 December 2021).
- European Commission. Commission Directive (EC) No 2006/86/EC of 24 October 2006 implementing Directive 2004/23/EC of the European parliament and of the Council as regards traceability requirements, notification of serious adverse reactions and events and certain technical requirements for the coding, processing, preservation, storage and distribution of human tissues and cells. Off. J. Eur. Union 2006, L294, 32–50. Available online: http://data.europa.eu/eli/dir/2006/86/oj (accessed on 1 December 2021).
- European Directorate for the Quality of Medicines & Healthcare. European Committee (Partial Agreement) on organ transplantation (CD-P-TO). In Guide to the Quality and Safety of Tissues and Cells for Human Application, 4th ed.; EDQM: Strasbourg, France, 2019; ISBN 978-92-871-8945-5.
- Federal Assembly of the Swiss Confederation. Federal Act on Medicinal Products and Medical Devices (Therapeutic Products Act, TPA). SR 812.21. 2000. Available online: https://fedlex.data.admin.ch/eli/cc/2001/422 (accessed on 1 December 2021).
- Federal Assembly of the Swiss Confederation. Federal Act on the Transplantation of Organs, Tissues and Cells (Transplantation Act). SR 810.21. 2004. Available online: https://fedlex.data.admin.ch/eli/cc/2007/279 (accessed on 1 December 2021).
- Center for Biologics Evaluation and Research, US Food and Drug Administration. Guidance for Industry on Characterization and Qualification of Cell Substrates and other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications; FDA: Silver Spring, MD, USA, 2010.
- European Medicines Agency. ICH Topic Q 5 A (R1). Quality of Biotechnological Products: Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin; CPMP/ICH/295/95; European Medicines Agency: Amsterdam, The Netherlands, 1997.
- European Medicines Agency. ICH Topic Q 5 D. Quality of Biotechnological Products: Derivation and Characterization of Cell Substrates Used for Production of Biotechnological/Biological Products; CPMP/ICH/294/95; European Medicines Agency: Amsterdam, The Netherlands, 1998.
- World Health Organization. Application of Hazard Analysis and Critical Control Point (HACCP) Methodology to Pharmaceuticals; WHO Technical Report Series No. 908; World Health Organisation: Geneva, Switzerland, 2003.
- Dahiya, S.; Khar, R.; Chhikara, A. Opportunities, challenges and benefits of using HACCP as a quality risk management tool in the pharmaceutical industry. Qual. Assur. J. 2009, 12, 95–104.
- European Directorate for the Quality of Medicines & Healthcare. General Chapter 5.2.3. Cell Substrates for the Production of Vaccines for Human Use. In European Pharmacopoeia 9.0; EDQM: Strasbourg, France, 2017.
- Laurent, A.; Scaletta, C.; Michetti, M.; Hirt-Burri, N.; de Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. GMP tiered cell banking of non-enzymatically isolated dermal progenitor fibroblasts for allogenic regenerative medicine. In Stem Cells and Good Manufacturing Practices: Methods and Protocols; Kursad, T., Ed.; Springer Science+Business Media: New York, NJ, USA, 2020.
- European Directorate for the Quality of Medicines & Healthcare. General Chapter 5.2.1. Terminology Used in Monographs on Biological Products. In European Pharmacopoeia 9.0; EDQM: Strasbourg, France, 2017.
- Zhang, K.; Na, T.; Wang, L.; Gao, Q.; Yin, W.; Wang, J.; Yuan, B.Z. Human diploid MRC-5 cells exhibit several critical properties of human umbilical cord-derived mesenchymal stem cells. Vaccine 2014, 32, 6820–6827.
- Hayflick, L.; Plotkin, S.A.; Norton, T.W.; Koprowski, H. Preparation of poliovirus vaccines in a human fetal diploid cell strain. Am. J. Hyg. 1962, 75, 240–258.
- Jordan, I.; Sandig, V. Matrix and backstage: Cellular substrates for viral vaccines. Viruses 2014, 6, 1672–1700.
- European Parliament and Council. Directive 2001/83/EC on the Community code relating to medicinal products for human use. Off. J. Eur. Union 2001, L311, 67–128. Available online: http://data.europa.eu/eli/dir/2001/83/oj (accessed on 1 December 2021).
- European Parliament and Council. Regulation (EC) No 726/2004 laying down Community procedures for the authorization and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency. Off. J. Eur. Union 2004, L136, 1–33. Available online: http://data.europa.eu/eli/reg/2004/726/oj (accessed on 1 December 2021).
- Klykens, J.; Pirnay, J.P.; Verbeken, G.; Giet, O.; Baudoux, E.; Jashari, R.; Vanderkelen, A.; Ectors, N. Cleanrooms and tissue banking how happy I could be with either GMP or GTP? Cell. Tissue Bank. 2013, 14, 571–578.
- European Commission. Commission Directive (EC) No 2003/94/EC of 8 October 2003 laying down the principles and guidelines of good manufacturing practice in respect of medicinal products for human use and investigational medicinal products for human use. Off. J. Eur. Union 2003, L262, 22–26. Available online: http://data.europa.eu/eli/dir/2003/94/oj (accessed on 1 December 2021).
- European Medicines Agency. ICH Topic Q 7. Good Manufacturing Practice for Active Pharmaceutical Ingredients; CPMP/ICH/4106/00; European Medicines Agency: Amsterdam, The Netherlands, 2000.
- European Medicines Agency. Re-Establishment of Working Seeds and Working Cell Banks Using TSE Compliant Materials; EMEA/22314/02; European Medicines Agency: Amsterdam, The Netherlands, 2002.
- European Medicines Agency. Guideline on the Use of Porcine Trypsin Used in the Manufacture of Human Biological Medicinal Products; EMA/CHMP/BWP/814397/2011; European Medicines Agency: Amsterdam, The Netherlands, 2014.
- European Medicines Agency. Note for Guidance on the Use of Bovine Serum in the Manufacture of Human Biological Medicinal Products; CPMP/BWP/1793/02; European Medicines Agency: Amsterdam, The Netherlands, 2003.
- European Medicines Agency. ICH Topic Q 5 E. Note for Guidance on Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process; CPMP/ICH/5721/03; European Medicines Agency: Amsterdam, The Netherlands, 2005.
- European Medicines Agency. ICH Topic Q 9. Guideline on Quality Risk Management; EMA/CHMP/ICH/24235/2006; European Medicines Agency: Amsterdam, The Netherlands, 2015.
- European Medicines Agency. ICH Topic Q 10. Guideline on Pharmaceutical Quality System; EMA/CHMP/ICH/214732/2007; European Medicines Agency: Amsterdam, The Netherlands, 2015.
- US Food and Drug Administration. Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals; FDA: Silver Spring, MD, USA, 1993.
- European Medicines Agency. ICH Topic Q 6 B. Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products; CPMP/ICH/365/96; European Medicines Agency: Amsterdam, The Netherlands, 1999.
- European Medicines Agency. ICH Topic Q 5 C. Stability Testing of Biotechnological/Biological Products; CPMP/ICH/138/95; European Medicines Agency: Amsterdam, The Netherlands, 1996.
- European Medicines Agency. Guideline on Risk Management Systems for Medicinal Products for Human Use; EMEA/CHMP/96268/2005; European Medicines Agency: Amsterdam, The Netherlands, 2005.
- European Parliament and Council. Regulation (EC) No 1394/2007 on Advanced Therapy Medicinal Products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004. Off. J. Eur. Union 2007, L324, 121–137. Available online: http://data.europa.eu/eli/reg/2007/1394/oj (accessed on 1 December 2021).
- Committee for Medicinal Product for Human Use. Guideline on Human Cell-Based Medicinal Products; EMEA/CHMP/410869/2006; European Medicines Agency: Amsterdam, The Netherlands, 2008.
- European Commission. EudraLex, the Rules Governing Medicinal Products in the European Union, Volume 4, Good Manufacturing Practice, Guidelines of 22.11.2017, Good Manufacturing Practice for Advanced Therapy Medicinal Products. 2017. Available online: https://ec.europa.eu/health/documents/eudralex/vol-4_en (accessed on 1 December 2021).
- European Medicines Agency. Creutzfeldt-Jakob Disease and Advanced Therapy Medicinal Products; EMA/CHMP/BWP/353632/2010; European Medicines Agency: Amsterdam, The Netherlands, 2011.
- Hunsberger, J.; Harrysson, O.; Shirwaiker, R.; Starly, B.; Wysk, R.; Cohen, P.; Allikson, J.; Yoo, J.; Atala, A. Manufacturing road map for tissue engineering and regenerative medicine technologies. Stem Cells Transl. Med. 2015, 4, 130–135.
- Addor, V.; Narring, F.; Michaud, P.A. Abortion trends 1990–1999 in a Swiss region and determinants of abortion recurrence. Swiss Med. Wkly. 2003, 133, 219–226.
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194.
- Laurent, A.; Simon, J.P.; Hirt-Burri, N.; Raffoul, W.; Applegate, L.A.; de Buys Roessingh, A.S. GMP-grade allogeneic musculoskeletal primary progenitor cell types: Standardized candidates for general or pharmacopeial monograph elaboration. J. Transl. Sci. 2020, 7, 1–3.
- Jeannerat, A.; Peneveyre, C.; Armand, F.; Chiappe, D.; Hamelin, R.; Scaletta, C.; Hirt-Burri, N.; de Buys Roessingh, A.; Raffoul, W.; Applegate, L.A.; et al. Hypoxic incubation conditions for optimized manufacture of tenocyte-based active pharmaceutical ingredients of homologous standardized transplant products in tendon regenerative medicine. Cells 2021, 10, 2872.
- Bari, E.; Ferrarotti, I.; Torre, M.L.; Corsico, A.G.; Perteghella, S. Mesenchymal stem/stromal cell secretome for lung regeneration:. The long way through “pharmaceuticalization” for the best formulation. J. Control. Release 2019, 309, 11–24.
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020, 29, 747–754.
- Chemali, M.; Laurent, A.; Scaletta, C.; Waselle, L.; Simon, J.P.; Michetti, M.; Brunet, J.F.; Flahaut, M.; Hirt-Burri, N.; Raffoul, W.; et al. Burn center organization and cellular therapy integration: Managing risks and costs. J. Burn Care Res. 2021, 42, 911–924.
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354 (Suppl. 1), S32–S34.
- European Directorate for the Quality of Medicines & Healthcare. European Pharmacopoeia 9.0 Monograph 2323. Human Hematopoietic Stem Cells; EDQM: Strasbourg, France, 2017.
- Verbeken, G.; Draye, J.P.; Fauconnier, A.; Vanlaere, I.; Huys, I.; De Corte, P.; Verween, G.; Pascual, B.; Delmotte, N.; Pierlot, A.; et al. The magistral preparation of Advanced Therapy Medicinal Products (ATMPs). J. Surg. Pract. 2020, 2, 16.
- Pirnay, J.P.; Verbeken, G.; Ceyssens, P.J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The magistral phage. Viruses 2018, 10, 64.
- Bicudo, E.; Brass, I.; Carmichael, P.; Farid, S. The UK’s emerging regulatory framework for point-of-care manufacture: Insights from a workshop on advanced therapies. Cell Gene Ther. Insights 2021, 7, 1005–1015.
- Hausherr, T.C.; Nuss, K.; Thein, E.; Applegate, L.A.; Pioletti, D.P. Human bone progenitor cells for clinical application: What kind of immune reaction does fetal xenograft tissue trigger in immunocompetent rats? Cell Transplant. 2017, 26, 879–890.
- Laurent, A.; Hirt-Burri, N.; Amiot, C.; Scaletta, C.; Applegate, L.A.; de Buys Roessingh, A.S. Primary progenitor muscle cells for regenerative medicine: Standardization of therapeutic protocols and optimized in vivo murine model for volumetric muscle loss. AJBSR 2020, 8, 143–153.
- Laurent-Applegate, L.A. Preparation of Parental Cell Bank from Foetal Tissue. European Patent No 2,732,030,B1, 3 July 2012. Available online: https://patents.google.com/patent/US9434923B2/en (accessed on 1 December 2021).
