
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rene LAFONT | + 10482 word(s) | 10482 | 2021-11-29 07:02:04 | | | |
| 2 | Nora Tang | -80 word(s) | 10402 | 2022-01-28 09:39:17 | | | | |
| 3 | Nora Tang | -9811 word(s) | 591 | 2022-04-13 11:28:17 | | |
Video Upload Options
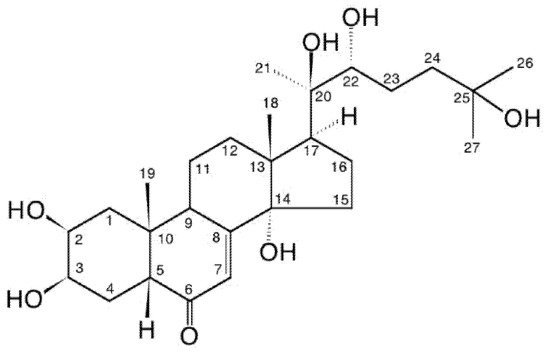
Ecdysteroid: member of a class of polyhydroxylated steroids found in invertebrate animals (zooecdysteroids; moulting hormones), plants (phytoecdysteroids) and fungi (mycoecdysteroids). Over 500 structural analogues are currently known. Biosynthetically, they derive from C27-, C28- or C29-sterols. The most frequently encountered analogue (in arthropods and plants) is 20-hydroxyecdysone (2β,3β,14α,20R,22R,25-hexahydroxycholest-7-en-6-one). In arthropods, ecdysteroids occur universally and regulate development by inducing moulting and reproduction, where their action is mediated by high-affinity binding to an intracellular member of the class of nuclear receptor (NR) proteins (ecdysteroid receptor; EcR) dimerised with a second NR (USP/RxR). This receptor complex binds to specific DNA promoter sites and regulates gene expression. In plants, ecdysteroids are a class of secondary compounds, occurring in varying amounts in certain species, but not all in others. Phytoecdysteroids are believed to contribute to the reduction of invertebrate predation by acting as feeding deterrents or endocrine disruptors. Ecdysteroids also possess a wide range of positive pharmacological effects in mammals, where the mode of action involves moderate-affinity binding to plasma-membrane-bound receptors and not interaction with the classical NRs for vertebrate steroid hormones.
References
- Koolman, J. (Ed.) Ecdysone: From Chemistry of Mode of Action; Thieme Verlag: Stuttgart, Germany, 1989; 482p.
- Dinan, L.; Savchenko, T.; Whiting, P. On the distribution of phytoecdysteroids in plants. Cell Mol. Life Sci. 2001, 58, 1121–1132.
- Dinan, L.; Harmatha, J.; Volodin, V.; Lafont, R. Phytoecdysteroids: Diversity, biosynthesis and distribution. In Ecdysone: Structures and Functions; Smagghe, G., Ed.; Springer Science & Business Media B.V.: Berlin, Germany, 2009; pp. 3–45.
- Lafont, R.; Harmatha, J.; Marion-Poll, F.; Dinan, L.; Wilson, I.D. The Ecdysone Handbook, 3rd ed.; Cybersales: Prague, Czech Republic, 2002; Available online: http://ecdybase.org/ (accessed on 1 April 2021).

