Ecdysteroid: member of a class of polyhydroxylated steroids found in invertebrate animals (zooecdysteroids; moulting hormones), plants (phytoecdysteroids) and fungi (mycoecdysteroids). Over 500 structural analogues are currently known. Biosynthetically, they derive from C27-, C28- or C29-sterols. The most frequently encountered analogue (in arthropods and plants) is 20-hydroxyecdysone (2β,3β,14α,20R,22R,25-hexahydroxycholest-7-en-6-one). In arthropods, ecdysteroids occur universally and regulate development by inducing moulting and reproduction, where their action is mediated by high-affinity binding to an intracellular member of the class of nuclear receptor (NR) proteins (ecdysteroid receptor; EcR) dimerised with a second NR (USP/RxR). This receptor complex binds to specific DNA promoter sites and regulates gene expression. In plants, ecdysteroids are a class of secondary compounds, occurring in varying amounts in certain species, but not all in others. Phytoecdysteroids are believed to contribute to the reduction of invertebrate predation by acting as feeding deterrents or endocrine disruptors. Ecdysteroids also possess a wide range of positive pharmacological effects in mammals, where the mode of action involves moderate-affinity binding to plasma-membrane-bound receptors and not interaction with the classical NRs for vertebrate steroid hormones.
This Entry is dedicated to the memory of Prof. Dr. Jan Koolman (1943–2021), a passionate ‘ecdysonist’ and an excellent teacher.
Ecdysteroids are a family of invertebrate steroid hormones that are involved in the regulation of moulting, development and reproduction
1. Introduction
This Entry is dedicated to the memory of Prof. Dr. Jan Koolman (1943–2021), a passionate ‘ecdysonist’ and an excellent teacher.
Ecdysteroids are a family of invertebrate steroid hormones that are involved in the regulation of moulting, development and reproduction
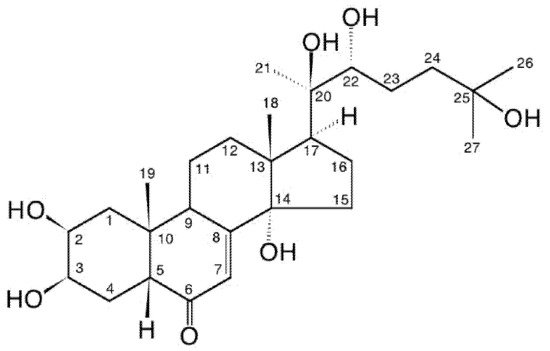
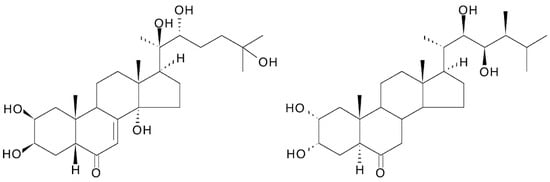
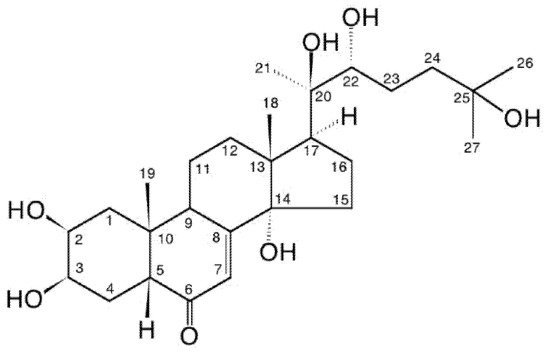
[1]. They differ significantly in their structure from vertebrate steroid hormones since they are characteristically polyhydroxylated, generally retain the full sterol carbon skeleton, possess a 14α-hydroxy-7-en-6-one chromophoric group located in the B-ring and possess an A/B-cis-ring junction. Thus, they markedly differ from vertebrate steroid hormones in their polarity, bulk and shape, and there is no convincing evidence that ecdysteroids interact with nuclear receptors for the vertebrate steroids in mammals. 20-hydroxyecdysone (20E; Figure 1) is the major biologically active form in insects, but other analogues act as biosynthetic intermediates (e.g., 2-deoxyecdysone), pro-hormones (ecdysone and/or 3-dehydroecdysone), metabolites (e.g., 20,26-dihydroxyecdysone) or storage forms (e.g., ecdysteroid phosphates). Other ecdysteroids may be hormonally active in other invertebrates (e.g., ponasterone A in crustaceans). In accord with their hormonal role, the concentrations of ecdysteroids found in arthropods and other invertebrates are generally rather low (nM to μM), with the storage forms being present in the highest amounts where they occur. . They differ significantly in their structure from vertebrate steroid hormones since they are characteristically polyhydroxylated, generally retain the full sterol carbon skeleton, possess a 14α-hydroxy-7-en-6-one chromophoric group located in the B-ring and possess an A/B-cis-ring junction. Thus, they markedly differ from vertebrate steroid hormones in their polarity, bulk and shape, and there is no convincing evidence that ecdysteroids interact with nuclear receptors for the vertebrate steroids in mammals. 20-hydroxyecdysone (20E; Figure 1) is the major biologically active form in insects, but other analogues act as biosynthetic intermediates (e.g., 2-deoxyecdysone), pro-hormones (ecdysone and/or 3-dehydroecdysone), metabolites (e.g., 20,26-dihydroxyecdysone) or storage forms (e.g., ecdysteroid phosphates). Other ecdysteroids may be hormonally active in other invertebrates (e.g., ponasterone A in crustaceans). In accord with their hormonal role, the concentrations of ecdysteroids found in arthropods and other invertebrates are generally rather low (nM to μM), with the storage forms being present in the highest amounts where they occur.
Figure 1. 20-hydroxyecdysone (20E; β-ecdysone; crustecdysone; ecdysterone; polypodine A; isoinokosterone; CAS 5289-74-7; IUPAC 2β,3β,14α,20R,22R,25-hexahydroxy-5β-cholest-7-en-6-one).
In addition to ecdysteroids occurring in invertebrates (zooecdysteroids), they are also present in certain plant species, such as phytoecdysteroids, where they are believed to contribute to the deterrence of invertebrate predators. They are present in detectable amounts in the seeds of 5–6% of investigated plant species and in leaves of an even greater proportion of species [2]. Concentrations vary from just detectable to high, depending on the species, the plant part and the stage of development, accounting for 1–2% of dry weight in high accumulators. Phytoecdysteroid profiles may vary from simple (the presence of one or two major components) through intermediate (a mixture of major and minor components) to complex (a cocktail of many analogues) [3]. 20E is the most frequently encountered phytoecdysteroid and very frequently is the major phytoecdysteroid present in the plant. Currently, 537 natural ecdysteroid analogues have been identified (Ecdybase [4]), most of which have been isolated only from plants, probably in large part because of the higher concentrations found in plant sources; some are found in invertebrates and plants, and a few have only, so far, been detected in invertebrates.
2. Zooecdysteroids Are Arthropod Hormones
The first ecdysteroid was isolated in 1954 by Butenandt and Karlson [5] from pupae of the silkworm Bombyx mori using a multi-step process (Figure 2), relying on an insect bioassay to detect moult-inducing activity. This compound was named “ecdysone”, the structure of which was established only eleven years later [6]. It turned out that ecdysone was, in fact, a precursor of the active hormone, 20-hydroxyecdysone (20E; 20-hydroxyecdysone (20E; β-ecdysone; crustecdysone; ecdysterone; polypodine A; isoinokosterone; CAS 5289-74-7; IUPAC 2β,3β,14α,20R,22R,25-hexahydroxy-5β-cholest-7-en-6-one).
Prof. Peter Karlson and his laboratory in Marburg (Germany) where 500, then 1000 kg of silkworm pupae were processed to isolate pure ecdysone (Photographs courtesy of Prof. Jan Koolman, University of Marburg).
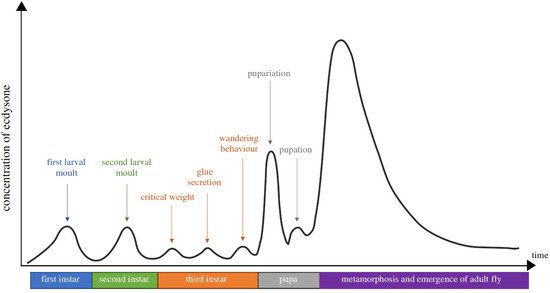
It was subsequently established that 20E was the moulting hormone of all arthropods (i.e., insects, crustaceans and arachnids). Ecdysone is produced in a cyclical way by moulting glands, and each peak induces a moult (Figure 3).
Figure 3. The developmental stages and ecdysteroid titres in Drosophila melanogaster. The X-axis is a timeline of the developmental stage, while the Y-axis represents the levels of ecdysteroid titre. Each of the ecdysteroid pulses triggers, respectively, embryogenesis, moulting, pupariation and metamorphosis. The ecdysteroid titre is expressed as 20E equivalents in whole-body homogenates (from Kannangara et al. [7]).
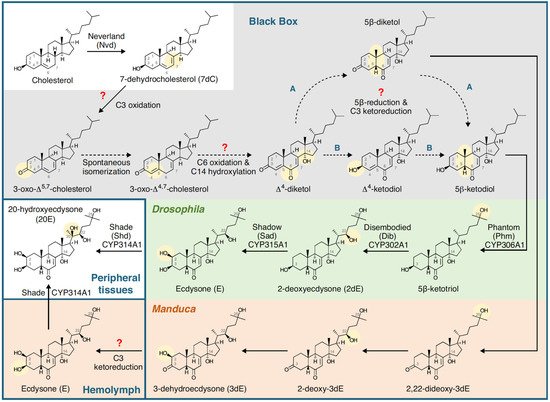
20E’s role is not restricted to larval life as it also controls metamorphosis; in adults, it controls reproduction, but in this case, it is produced by the gonads [8][9]. It controls developmental or reproductive seasonal arrests (diapauses [10]). 20E is produced from cholesterol through a multi-step process that is not yet fully elucidated [7][11][12] (Figure 4).
Figure 4. Summary of the biosynthetic pathway for 20-hydroxyecdysone in Drosophila melanogaster (Diptera) and Manduca sexta (Lepidoptera). Insects are incapable of synthesising sterols, so cholesterol or phytosterols have to be obtained in the diet. The early part of the pathway takes place in the ring gland (Drosophila) or prothoracic gland (Manduca), and the prohormones (3dE and/or E) are released into the haemolymph and converted to 20E (the major, hormonally active ecdysteroid) in peripheral tissues. Note that the C6-oxidation and 14α-hydroxylation reactions are as yet uncharacterised (the ‘Black Box’). Figure reproduced from Pan et al. [13] with permission.
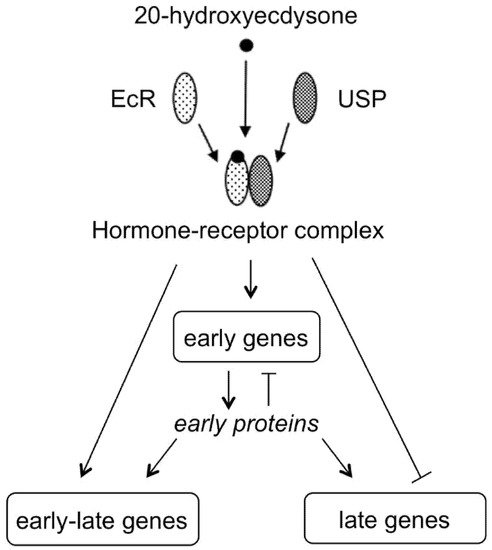
Understanding the mechanism of action of 20E has benefitted from a unique biological model, based on the giant polytene chromosomes present in salivary glands of cyclorrhaphous Diptera, that allows direct observation of sites of active DNA transcription as “puffs”, i.e., specific zones where chromatin is decondensed, allowing its transcription to mRNA [14]. This biological model allowed description of the induction by 20E of a specific sequence of puffs, and this was a key finding for understanding the mechanism of action of steroid hormones through their binding to nuclear receptors and inducing a sequence of gene activations (Figure 5) [15][16].
Figure 5. 20E binds to a nuclear receptor (EcR) that forms a heterodimer with USP protein to activate the transcription of early genes that encode for transcription factors. This initiates a sequence of gene activations leading to the transcription of late genes that requires the withdrawal of the hormone (modified from Thummel [17]).
In addition to ecdysteroids occurring in invertebrates (zooecdysteroids), they are also present in certain plant species, such as phytoecdysteroids, where they are believed to contribute to the deterrence of invertebrate predators. They are present in detectable amounts in the seeds of 5–6% of investigated plant species and in leaves of an even greater proportion of species
3. Phytoecdysteroids Are Plant Secondary Metabolites
3.1. Discovery
The presence of ecdysteroids in plants was first determined by several groups almost simultaneously, shortly after the final unambiguous elucidation of the structure of ecdysone from Bombyx mori pupae [6], in various plant species: Podocarpus nakaii [18], Achyranthes fauriei [19], Podocarpus elatus [20], Polypodium vulgare [21][22] and Pteridium aquilinum [23]. All but one of these reports concerned the isolation of 20-hydroxyecdysone, which had also been identified, along with ecdysone, as a more minor component from B. mori pupae [24], and two reports also identified close analogues—ponasterone A (25-deoxy-20-hydroxyecdysone; ponA), which is amongst the most biologically active ecdysteroids in arthropod systems, ponasterone B (2,3-di-epi-ponA), ponasterone C (5β,24S-dihydroxyponA) [18][25][26] and inokosterone [19]. However, a very significant finding of these early studies was that ecdysteroids, when they occur in plants, occur at much higher concentrations than in insects, such that the same amount of 20E as was isolated from 500 kg of silkworm pupae could be isolated from less than 1 g (d.w.) of an ecdysteroid-containing plant species. This relatively high accumulation facilitated the identification of further phytoecdysteroids and prompted the search for other ecdysteroid-accumulating species in the plant world [27][28][29].
3.2. Distribution in the Plant World
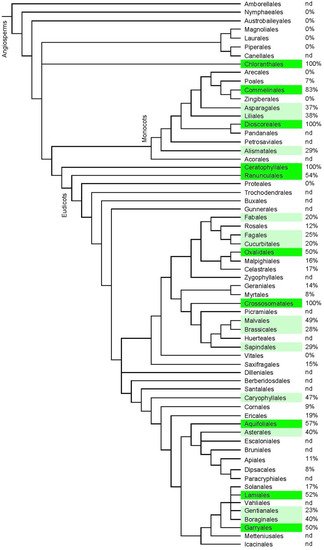
A wide distribution of ecdysteroid-containing species has been determined, including in algae, ferns [30], gymnosperms, angiosperms, and fungi (mycoecdysteroids; [31][32]), but only 5–6% of the seeds/spores of investigated species of plants contain detectable concentrations by sensitive bioassay/RIA methods [33], which results in big differences even within a single genus since some species can be high accumulators while others contain no detectable ecdysteroid. Some families of angiosperms contain many ecdysteroid-positive species (Figure 6).
Figure 6. Indication of the frequency of ecdysteroid-containing species within the orders of angiosperm plants. The classification is according to APGIV (2016) [34], and the data concerning the presence or absence of phytoecdysteroids was taken from the “Compilation of the literature reports for the screening of vascular plants, algae, fungi and non-arthropod invertebrates for the presence of ecdysteroids” (Version 11; www.ecdybase.com), which provides data on 2282 angiosperm species. The data is qualitative (i.e., present or absent) and does not necessarily reflect the occurrence of high accumulators. The % frequencies of ecdysteroid-containing species amongst the assessed species in each order are indicated in the right-hand column; nd = no data available. Green colour: 50–100%; light green: 20–49% of investigated species.
Examination of the leaves of randomly selected species demonstrated that ca. 40% of the species contained at least low levels of ecdysteroids, and examination of many individuals of an “ecdysteroid-negative” species (Arabidopsis thaliana) found individual plants that contained low levels of ecdysteroids, even if the majority were negative
[2]. Concentrations vary from just detectable to high, depending on the species, the plant part and the stage of development, accounting for 1–2% of dry weight in high accumulators. Phytoecdysteroid profiles may vary from simple (the presence of one or two major components) through intermediate (a mixture of major and minor components) to complex (a cocktail of many analogues) . These three lines of evidence strongly suggest that most, if not all, plants retain the genetic capacity to synthesise ecdysteroids but that the expression of the biosynthetic pathway(s) is down-regulated in most species and at certain stages of development. It is possible that this conclusion can be extrapolated to other classes of steroids/triterpenoids among the secondary defence compounds produced by plants (e.g., bufadienolides, cucurbitacins, steroidal saponins, withanolides).
3.3. Range of Analogues and Biosynthesis
The phytochemical investigation of a wide range of plant species has resulted in the identification of a large number of ecdysteroid analogues. Information about all (currently 537) the published naturally-occurring (zoo-, phyto-, myco-)ecdysteroid structures and their spectroscopic, physico-chemical and biological activity data and relevant references are compiled in Ecdybase (www.ecdybase.org: [4]). This website is continuously curated to give an up-to-date overview of the topic. Phytoecdysteroids comprise free ecdysteroids and polar (glycosides, sulphates) and non-polar (e.g., acetates, benzoates, coumarates) conjugates. The huge diversity of phytoecdysteroid molecular structures indicates that biosynthesis in plants occurs as a combinatorial system of a network of enzymes without strong substrate specificity, which could result in over 1000 possible permutations of structure from a small number of early intermediates (14α-hydroxy-6-oxo-7-ene C27/28/29-sterols [35]). The ecdysteroid profile produced is then determined by the flux through this network and the relative expression, activity and regulation of the various enzymes (e.g., hydroxylases, oxidases, epimerases, acyl transferases, glycose transferases).
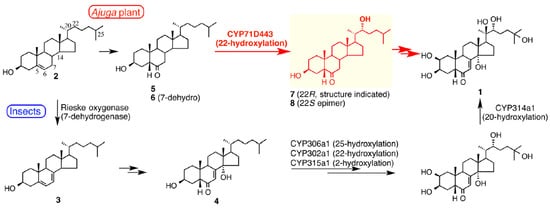
The biosynthetic pathway(s) in plants, just like that in arthropods, has not yet been fully elucidated, but the biosynthesis appears to differ mechanistically from that in animals (Figure 7), and maybe also differ within types of plant (e.g., between angiosperms and ferns;
[3][35]. 20E is the most frequently encountered phytoecdysteroid and very frequently is the major phytoecdysteroid present in the plant. Currently, 537 natural ecdysteroid analogues have been identified (Ecdybase ), raising the question whether phytoecdysteroid biosynthesis has a monophyletic or polyphyletic origin. However, our very incomplete knowledge of the biochemical routes, enzymology and regulation of phytoecdysteroid biosynthesis seriously hampers the answering of this and many other important questions.
Figure 7. Summaries of the biosynthetic pathways for 20-hydroxyecdysone (1) from cholesterol (2) in insects (lower route) and Ajuga reptans var. atropurpurea hairy roots (upper route), highlighting the earlier involvement of a 22-hydroxylated intermediate (7) in the biosynthesis in Ajuga. Figure reproduced from Tsukagoshi et al. [36] (with permission).
In addition to providing reference compounds for biochemical studies, the extensive range of ecdysteroid analogues provides an excellent natural resource for SAR studies in arthropods (reviewed in [37]) and mammals [38]. The chemical space provided by the natural ecdysteroids is being extended by the preparation and biological assessment of numerous semi-synthetic analogues (e.g., [37][38][39][40][41]).
3.4. High-Accumulating Species
The identification of highly accumulating species (≥1% d.w.) is important for the isolation of ecdysteroid analogues for biochemical/pharmacological assessment and clinical trials (currently essentially 20E only). Table 1 lists the best sources of 20E identified so far. It is worth mentioning that only ca. 2% of all plant species have, as yet, been assessed in any way for the presence of ecdysteroids [42][43][44], so there may be other species that outperfom those listed in Table 1. However, the 6000 or so species that have so far been assessed (see [45] for a compilation of the analysed species) represent those species that are more readily accessible, meaning that any further extensive survey would require a large amount of work to collect and confirm the identity of the plant material.
Table 1. Some plant sources containing significant amounts of 20-hydroxyecdysone; levels ≥1% (w/w) are indicated in bold. The original literature references concerning the ecdysteroids present in these species can be found in Ecdybase
[4]), most of which have been isolated only from plants, probably in large part because of the higher concentrations found in plant sources; some are found in invertebrates and plants, and a few have only, so far, been detected in invertebrates. .
| Species |
Family |
Plant Part |
Amount (mg/g) |
| Achyranthes japonica |
Amaranthaceae |
dry leaves |
2 |
| Ajuga chamæpitys |
Labiatae |
dry whole plant |
1.6 |
| Ajuga decumbens |
Labiatae |
whole plant |
1.3 |
| Ajuga japonica |
Labiatae |
dry leaves |
2 |
| Cyanotis arachnoidea |
Commelinaceae |
dry whole plant |
12 |
52][53] and growth and heterocyst formation in the cyanobacterium Nostoc [54]. Such effects may be related to the stimulation of the activity of the photosynthetic enzyme RuBisCO by 20E, AjuC and PolB, demonstrated in vitro for the enzyme isolated from Tetragonia tetragonoides (New Zealand spinach; [55]). Thus, whether ecdysteroids have non-defence purposes in plants remains an open question at present.
3.7. Allelochemical Role
Phytoecdysteroids may act as feeding deterrents [56][57] and/or endocrine disruptors for insects [58][59][60][61][62][63][64][65][66] and soil nematodes [67]. However, they are not universally active because certain insects are adapted to cope with phytoecdysteroids; not all insects possess taste receptors recognising the presence of ecdysteroids in plants, and some insects possess very effective detoxification mechanisms to deal with ingested ecdysteroids. On the other hand, insects that do not encounter phytoecdysteroids in their host plants can be remarkably susceptible to them, such that they would rather starve than consume host plant material treated with ecdysteroids or consume it and then undergo serious/lethal developmental abnormalities (Table 2).
Table 2. The susceptibility of assessed invertebrate species to ingested 20-hydroxyecdysone relative to the fate of the compound on ingestion.
| Species |
Order |
Common Name |
Mono-/Oligo-/Polyphagous |
Effects of Feeding 20E |
Resistant or Susceptible |
Fate of Ingested 20E |
Refs. |
| INSECTS |
| Achaea janata |
|
Castor semi-looper |
Polyphagous |
Inhibition of adult emergence |
Susceptible |
|
[68] |
| Acherontia atropos |
Lepidoptera |
Death’s head hawkmoth |
Polyphagous (some host plants ecdysteroid-positive) |
None at 400 ppm |
Resistant |
Excretes 20E unmetabolised |
[63] |
dry roots |
| Acrolepiopsis assectella |
Lepidoptera |
Leek moth |
Oligophagous on the Alliaceae (ecdysteroid-negative) |
Developmental disruption and death |
Susceptible |
|
[69] |
| Aglais urticae |
Lepidoptera |
Small tortoiseshell butterfly |
Monophagous (on nettles—ecdysteroid negative) |
Initial antifeedant effect, followed by reduced feeding and developmental disruption |
Susceptible |
Excretion of 20E and polar metabolites (no FA conjugates) |
[64] |
23 |
| Agrius convolvulae |
Lepidoptera |
Sweet potato hornworm |
Polyphagous |
None at 1600 ppm (but effects of E at 400 ppm) |
Resistant |
|
[70] |
Cyanotis vaga |
Commelinaceae |
dry leaves |
7 |
| Bombyx mori |
Lepidoptera |
Domestic silkworm |
Monphagous (on mulberry—ecdysteroid-negative) |
Enhanced developmental synchrony at very low concentrations. Feeding deterrence, developmental defects and death at higher concentrations |
Susceptible |
E is converted to 3-epiE and a sulphate conjugate |
[71][72][73][74][75] |
Dacrydium intermedium |
Podocarpaceae |
dry bark |
| Bradysia impatiens |
Diptera |
Darkwinged fungus gnat10 |
| Soil-dwelling mycophage |
Reduced survival |
Susceptible |
|
[76] |
Diploclisia glaucescens |
Menispermaceae |
dry stem |
| Chloridea (Heliothis/ Helicoverpa) virescens |
Lepidoptera |
Tobacco budworm32 |
| Polyphagous |
None at 1000 ppm |
Resistant |
Excretion of 20E 22-FA esters |
[73][77] |
Helleborus abchasicus |
Ranunculaceae |
dry aerial parts |
2.4 |
| Cynthia cardui |
Lepidoptera |
Painted lady butterfly |
Polyphagous (some host plants ecdysteroid-positive) |
Tolerates low levels, but increasing antifeedant effect at higher concentrations |
Intermediate |
Excretion of 20E, polar metabolites and minor apolar metabolites (no FA conjugates) |
64 |
Helleborus atrorubens |
Ranunculaceae |
dry aerial parts |
2.5 |
| Gryllus bimaculatus |
Orthoptera |
Two-spotted cricket |
Polyphagous |
|
|
Conversion to 14-deoxy20E and 20E 22-FA esters |
[78][79] |
Helleborus bocconei |
Ranunculaceae |
dry aerial parts |
2.6 |
| Heliothis (Helicoverpa) armigera |
Lepidoptera |
Cotton bollworm |
Polyphagous |
None at 50 μg E per insect |
Resistant |
E is excreted unchanged, mainly as E 22-palmitate and as 2/3-acetate |
[80] |
Helleborus cyclophyllus |
Ranunculaceae |
| Inachis io |
Lepidopteradry aerial parts |
2.7 |
| Peacock butterfly |
Oligophagous (host plants ecdysteroid-negative) |
Strong antifeedant effect, resulting in starvation |
Susceptible |
Excretion of 20E and polar metabolites (no FA conjugates) |
[64] |
Helleborus dumentorum |
Ranunculaceae |
dry aerial parts |
2.9 |
| Lacanobia oleracea |
Lepidoptera |
Tomato moth |
Polyphagous (some host plants ecdysteroid-positive) |
None at 400 ppm |
Resistant |
Conjugation to long-chain FAs and rapid excretion |
[62][63] |
Helleborus guttatus |
Ranunculaceae |
dry aerial parts |
2.7 |
| Helleborus multifidus |
Ranunculaceae |
dry aerial parts |
| Lobesia botrana |
Lepidoptera |
European grapevine moth |
Oligophagous (on ecdysteroid-negative plants) |
Enhanced mortality |
Susceptible |
|
[81] |
3.6 |
| Locusta migratoria |
Orthoptera |
Migratory locust |
Polyphagous |
None at 400 ppm |
Resistant |
Conversion to 20E 2-phosphate, 3-acetyl 20E 2-phosphate and 20E 3Ac |
[82][83][84] |
Helleborus niger |
Ranuculaceae |
dry plant or roots |
2.1 |
| Mamestra brassicae |
Lepidoptera |
Cabbage armyworm |
Polyphagous |
None at 800 ppm |
Resistant |
|
[70] |
Helleborus orientalis |
Ranunculaceae |
dry aerial parts |
4.5 |
| Manduca sexta |
Lepidoptera |
Tobacco hornworm |
Oligophagous on plants of the Solanaceae (some are ecdysteroid-positive) |
None at 800 ppm |
Resistant |
Conversion to 2- and 22-phosphates and 3-epi-20E |
[85][86] |
Helleborus viridis |
Ranunculaceae |
| Myzus persicae | dry aerial parts |
2.1 |
| Hemiptera |
Peach-potato aphid |
Polyphagous |
|
|
Converted to 22-glucoside |
[87] |
Ipomoea calonyction |
Convolvulaceae |
| Ostrinia nubilalis | seeds |
1.5 |
| Lepidoptera |
European corn borer |
Polyphagous |
Antifeedant effect |
Susceptible |
Excreted as FA conjugates |
[57][75][87] |
Lychnis chalcedonica |
Caryophyllaceae |
dry aerial parts |
7.9 |
| Pectinophora gossypiella |
Lepidoptera |
Pink bollworm |
Oligophagous (on ecdysteroid-negative plants) |
Growth inhibition and developmental disruption |
Susceptible |
|
[71] |
Lychnis flos-coculi |
Caryophyllaceae |
dry plant |
1.7 |
| Plodia interpunctella |
Lepidoptera |
Indian meal moth |
Polyphagous (on grains) |
Effects at 200 ppm |
Resistant to low to moderate concentrations of 20E |
Converted to 3-oxo- and 3-epi-derivatives, excreted in free form and conjugated to FAs |
[87][88][89] |
Microsorum membranifolium |
Polypodiaceae |
dry fronds |
2.1 |
| Spodoptera littoralis |
Lepidoptera |
Egyptian cotton leafworm |
Polyphagous (some host plants ecdysteroid-positive) |
None at 100 ppm; feeding deterrent for 1st instar larvae |
Resistant |
Excretion of 20E and 22-FA conjugates |
[60][75] |
dry rhizomes |
0.6 |
| Microsorum scolopendria |
Polypodiaceae |
dry fronds |
2.2 |
| dry rhizomes |
6.8 |
| Pandiaka involucra |
Amaranthaceae |
dry aerial parts |
3.0 |
| Podocarpus elatus |
Podocarpaceae |
dry bark |
4.5 |
| Tyria jacobae |
Lepidoptera |
Cinnabar moth |
Oligophagous (some host plants ecdysteroid-positive) |
Dose-dependent effects, tolerating low levels, but higher concentrations bring about developmental defects and death |
Intermediate |
Rapid excretion of 20E and polar and apolar metabolites (no FA conjugates) |
[64] |
| Nematodes |
| Heterodera avenae |
Nematoda |
Cereal cyst nematode |
Oligophagous on cereal crops (ecdysteroid-negative) |
Abnormal moulting, immobility and impaired development |
Susceptible |
|
[67] |
| Heterodera schlachtii |
Nematoda |
Sugarbeet cyst nematode |
Polyphagous |
Abnormal moulting, immobility and impaired development |
SusceptiblePolypodium vulgare |
Polypodiaceae |
dry rhizomes |
10 |
| Rhaponticum carthamoides |
Asteraceae |
dry fruits |
15 |
| Rhaponticum integrifolium |
Asteraceae |
dry flowers |
1.5 |
| Rhaponticum scariosum |
Asteraceae |
dry fruits |
13 |
| Serratula inermis |
Asteraceae |
dry fruits/flowers |
20 |
| dry leaves |
2.5 |
| Serratula tinctoria |
Asteraceae |
fresh plant |
2 |
Figure 13. Examples of dietary supplements containing (or claimed to contain) 20E.
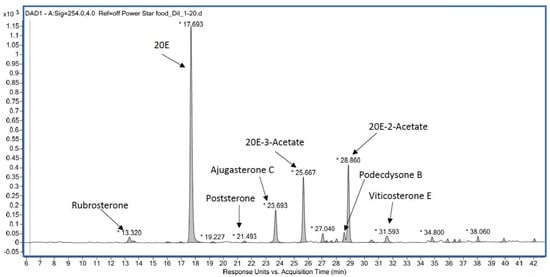
Such products generally contain much lower 20E amounts than announced [185]. They generally do not contain pure 20E but a more or less complex mixture (Figure 14), the profile of which may permit the identification of the source plant [186]. Moreover, the quality is not constant and may vary from batch to batch (unpublished observations).
Figure 14. Example of the RP-HPLC ecdysteroid profile of a dietary supplement from Powerstar Food (see Ecdybase.org for structures).
8. Ecdysteroids: A Multifaceted Human Medicine?
Research in this area has a long history and started soon after the structural identification of ecdysone (E) and 20-hydroxyecdysone (20E) from arthropods and the discovery of analogues in plants in 1965, when it was thought that the compounds might be useful invertebrate control agents. As a consequence, considerable effort was put into determining the toxicity and initial pharmacokinetic studies in mammals.
While Western scientists tended to focus on the metabolism and mode of action of ecdysteroids within invertebrates, Eastern European and Central Asian scientists continued to explore the pharmaceutical uses of ecdysteroids. The findings of these latter studies were mostly published in Slavic and Turkic languages, so their significance was not fully appreciated for quite some time [187]. In the past two decades, however, there has been a considerable increase in studies focusing on the effects and potential applications around the world, which has been enabled by the concomitant commercial availability of large amounts of pure 20E.
The prospect of using phytoecdysteroids as insect control agents in the field led to the undertaking of studies on their pharmacological effects on vertebrates/mammals. It was quickly apparent that their acute oral toxicity was very low (20E LD50 > 9 g/kg in rats [188]) and that they displayed interesting pharmacological effects [189]: (i) an antidiabetic effect [190]) and (ii) an anabolic effect, i.e., a stimulation of mRNA translation (“ribosomal amplifier” [191]. Then, the list of beneficial effects progressively increased (Table 3). Some of these effects were compared to those of plants used in traditional medicine, and the conclusion is that phytoecdysteroids display pleiotropic effects on mammals (Table 2; for a detailed list of references, see [170]).
Table 3. Pharmacological effects of ecdysteroids in mammals. See Dinan et al. [170] for a detailed bibliography.
| Major Effects Described for 20-Hydroxyecdysone or Related Molecules |
| Anabolic (muscle) |
Cardioprotective |
| Fat-reducing/Hypolipidaemic |
Neuromuscular protective |
| Anti-diabetic |
Neuroprotective |
| Anti-fibrotic |
Memory protective |
| Anti-inflammatory |
Liver protective |
| Antioxidant |
Lung protective |
| Anti-thrombotic |
Kidney protective |
| Vasorelaxant |
| Serratula xeranthemoides |
| Asteraceae |
| dry flower buds |
| 1.6 |
| dry inflorescences |
| 3.3 |
| fruit formation |
| 2.8 |
| seeds |
1.1 |
| Sesuvium portulacastrum |
Aizoaceae |
dry whole plant |
3.5 |
| |
[ |
Silene jenisseensis |
Caryophyllaceae |
dry whole plant |
35 |
| Silene nutans |
Caryophyllaceae |
dry whole plant |
2.7 |
| Silene otites |
Caryophyllaceae |
dry whole plant |
9.8 |
| dry inflorescences |
32.6 |
| dry stems/leaves |
5.9 |
| Silene praemixta |
Caryophyllaceae |
dry leaves |
25 |
| dry roots |
3.4 |
| dry inflorescences |
17 |
| Silene repens |
Caryophyllaceae |
dry whole plant |
12 |
| Tinospora cordifolia |
Menispermaceae |
dry stems |
0.25 |
| Vitex glabrata |
Verbenaceae |
dry stem bark |
18 |
3.5. Distribution within Ecdysteroid-Containing Plants
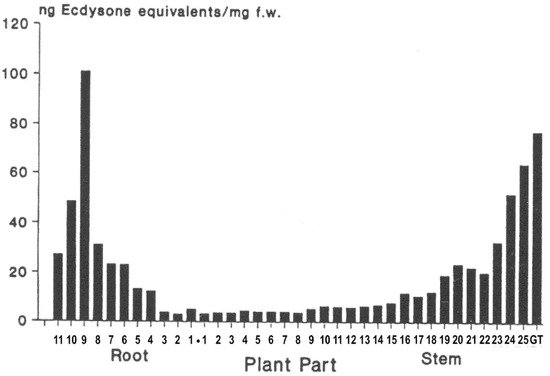
The distribution within a given ecdysteroid-containing plant specimen is far from homogeneous, with certain plant parts possessing higher concentrations and differing profiles than other parts. There is some evidence that this reflects differential accumulation and not only sites of biosynthesis. The accumulation seems to favour (protect) the parts of the plants that are important for the survival of the plant. Thus, in tender annual plants, such as spinach (Spinacia oleracea), the growing tips of the stem and the young leaves contain higher levels than the stem and root (Figure 8). Additionally, rhizomatous ecdysteroid-containing plants (e.g., Leuzea carthamoides) recycle the ecdysteroids from the aerial portions, which die back in the autumn, into the rhizome for re-use the following year. Flowers and seeds also often contain high concentrations of ecdysteroids, implying an important role in protecting these reproductive organs from predation.
Figure 8. Distribution of phytoecdysteroids in spinach: 1 cm sections of the roots and stems were extracted, and ecdysteroids were measured by RIA (taken from [33]—permission granted).
3.6. Physiological Roles in Plants?
Although the generally accepted function of phytoecdysteroids is in reducing invertebrate predation, there is literature concerning other possible roles in plants. It is not currently clear if these are true physiological roles or pharmacological effects. In particular, research has been performed to try to answer the question of whether phytoecdysteroids might have a hormonal role in plants themselves or interact with any of the nine classes of recognised plant hormones (abscisic acid, auxins, brassinosteroids, cytokinins, ethylene, gibberellins, jasmonates and strigolactones [46][47][48][49]). Generally, no definitive evidence for agonist, synergistic or antagonist activities could be found. 20E affects germination and stimulates shoot elongation in young tomato (Lycopersicum esculentum) seedlings [50], but this may reflect a more general effect of steroids since progesterone stimulates germination in maize (Zea mais; [51]). Ecdysteroids seem to stimulate the size and growth of the alga Chlorella vulgaris [ | ] |
| Meloidogyne javanica |
| Nematoda |
| Root-knot nematode/sugar cane eelworm |
| Polyphagous |
| Abnormal moulting, immobility and impaired development |
| Susceptible |
| |
| [ |
| 67 |
| ] |
| Pratylenchus neglectus |
| Nematoda |
| Root lesion nematode |
| Oligophagous (on ecdysteroid-negative plants) |
Abnormal moulting, immobility and impaired development |
Susceptible |
|
[67] |
3.8. Ecdysteroids for the Protection of Crop Species
A number of hypotheses have been put forward as to the role(s) of ecdysteroids in plants (see above), but the favoured hypothesis, for which evidence is accumulating, is that they serve to deter invertebrate predators (arthropods and nematodes) by acting as feeding deterrents or endocrine disruptors on ingestion. Since ecdysteroids are non-toxic to mammals, this makes them attractive for the protection of plant crop species. However, the hope in the early days of ecdysteroid research that ecdysteroids might be sprayed onto crop plants could not be realised because ecdysteroids are too difficult and expensive to chemically synthesise, are too unstable under field conditions (exposure to UV energy), do not readily penetrate arthropod cuticles and are rapidly metabolised in the guts of many invertebrate predator species on ingestion.
One alternative strategy that has been successfully commercially developed is the use of non-steroidal ecdysteroid analogues as insecticidal compounds (bisacylhydrazines; [90]) since these chemicals are easy to synthesise, non-polar and resistant to metabolism.
There is now renewed interest in strategies using ecdysteroids themselves by manipulating the ecdysteroid levels and/or profiles in crop plants. Since the compounds would be integral to the plant, there will not be the stability problems associated with surface spraying, but they are present for deterrence or upon ingestion. It has to be stated at the outset that very few plants used for human consumption are ecdysteroid-containing [43]. Spinach (S. oleracea) and quinoa (Chenopodium quinoa) are the only significant crop species containing ecdysteroids and then only at moderate levels. However, if the genes for ecdysteroid biosynthesis are present in all plant species (as explained above), it should be possible to activate their expression to elevate ecdysteroid levels in the plant, as a whole, in a developmentally controlled manner or in specific organs of the plant. Further, the biosynthetic flux could be directed to analogues with more desirable ADME properties, which, for example, are not so readily metabolised in the gut of predator species, while retaining hormonal activity.
3.9. Practical Uses of Phytoecdysteroids
3.9.1. In Sericulture
Silk production is practised as a cottage industry by farmers in many Asian countries. It has been found that application of low doses of ecdysteroids can improve the synchronisation of spinning and the yield of silk in the cocoon of Bombyx mori (domestic silkmoth) by 10–15% [91][92][93][94] and synchronisation of spinning in Antheraea mylitta (tasar silkworm; [95]). To provide a low-technology strategy, it has been proposed that suitable ecdysteroid-containing plant species (e.g., Achyranthes aspera, Chenopodium album, Coscinium fenestratum, Diploclisia glaucescens, Trianthema portulacastrum or Vitex negundo) can be collected by the farmers within their local area, and the plants can be extracted with water according to a standardised procedure to provide an ecdysteroid solution that can be sprayed onto leaves of the food-plants of the silkworm larvae (Morus alba [mulberry] for B. mori and Terminalia tomentosa [Indian laurel] for A. mylitta).
3.9.2. In Apiculture
Low doses of ecdysteroids can also improve the fecundity of the honey bees Apis mellifera [96].
3.9.3. In Aquaculture
A similar approach has been suggested for the synchronisation of development in prawns and reduction of the moulting period [97][98] and in the generation of soft-shelled crabs for the commercial market [99].
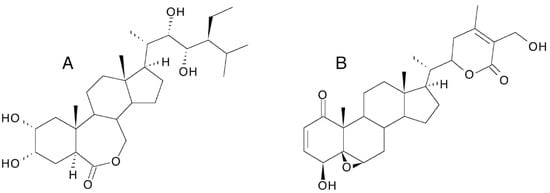
3.10. Similarity to Brassinosteroids
The structure of ecdysteroids is chemically related to that of brassinosteroids (plant growth hormones) in that both classes are polyhydroxylated steroids; two-dimensional representations of their structures make them look more similar than they really are. This has led to the assessment of brassinosteroids as ecdysteroid agonists or antagonists [100][101], where, at best, only very weak activity is found, and the question of whether ecdysteroids might have brassinosteroid-like activity. There is no evidence for the latter, and this would be biologically very inconvenient for an ecdysteroid-containing plant because brassinosteroids are present and active at very low concentration (nM), while ecdysteroids would be present in the μM—mM range and would swamp the brassinosteroid receptors if there was even a low cross-reactivity. If one closely considers the 3D structure of ecdysteroids and brassinosteroids (Figure 9), one sees that the distribution and orientation of the hydroxyl groups are different; the A/B-ring junction is cis in ecdysteroids and trans in brassinosteroids, resulting in a major difference in the 3D-shape of the molecules. Thus, natural members of the two classes of molecules can be expected to possess very different biochemical properties and biological activities.
Figure 9. Comparison of the structures of 20-hydroxyecdysone and castasterone (the brassinosteroid with the closest structural similarity to 20E).
A recent study [102] examined the reciprocal effects of 24-epi-brassinolide and 20E on shoot/root growth and endogenous brassinosteroid and ecdysteroid levels in Lepidium sativum (garden cress) seedlings (which contains detectable, but exceedingly low, levels of endogenous phytoecdysteroids) and found that exogenous 20E suppresses the levels of several endogenous brassinosteroids, especially brassinolide. However, synthetic ecdysteroid analogues that possess some brassinosteroid-like activity have been prepared [103]. The chemical similarity between the two classes of molecule allows cross-fertilisation in the semi-synthesis of analogues within each class since synthetic strategies and conditions can be similar [104][105].
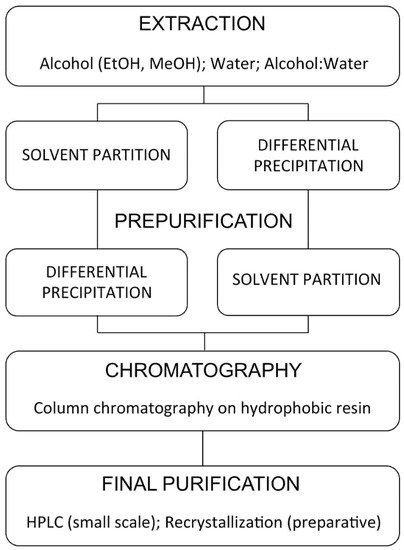
4. Methods of Purification, Analysis and Quantification of Ecdysteroids
Ecdysteroid extraction/purification is a multi-step process (Figure 10). Its rationale can be found in different reviews [38][106][107][108].
Figure 10. General procedures for ecdysteroid purification (modified after [107]).
4.1. Extraction
Ecdysteroids may be extracted from fresh or dried plant material. Drying is normally accomplished by lyophilisation or oven-drying (at ca. 50 °C), followed by grinding or powdering of the dried material to increase the surface area for extraction. In view of the significant polarity of 20E and related ecdysteroids, extraction of fresh or dried plant material is usually accomplished with alcohol (methanol or ethanol), perhaps in admixture with water (30–50%), typically at 10 mL extraction solvent to 1 g (repeated 2 or 3 times), with stirring and heating at ca. 60 °C over several hours to several days. The solubility of 20E in solvents on either side of methanol and ethanol on the polarity scale (acetone, ethyl acetate, acetonitrile, water) drops off considerably, so these are much poorer if high extraction yields are sought (and they normally are!). For sources that contain ecdysteroid glycosides, aqueous alcohol provides good extraction yields, but for sources containing non-polar conjugates (acetates, acetonides, glucosylferulates; [109]), pure alcohol is more effective. Prior extraction with a non-polar solvent (e.g., hexane) can be used to reduce lipophilic compounds and pigments. Heating at a moderate temperature with stirring or reflux (Soxhlet extraction) improves extraction rates and yields without artefactual modification of ecdysteroids. The presence of acetone (either as the extraction solvent or as a contaminant in the solvent) could result in the artefactual production of ecdysteroid acetonides, so it should be avoided. Ecdysteroid 2- or 3-acyl derivatives (especially acetates) equilibrate in the solution over time by the process of 2,3-acyl migration to give a mixture of the isomers, so they need to be processed quickly and stored dry to be able to accurately identify and quantify which are present. This equilibration is enhanced by the presence of acid and the same can also induce some dehydration of the 14-OH [106].
Supercritical fluid with critical carbon dioxide modified with methanol, ethanol or DMSO is an effective, high-yield extraction method for ecdysteroid-containing plant materials [110][111][112][113]. The method is not generally used, probably because it requires the extensive prior optimisation of parameters (pressure, temperature, % of modifier, extraction time and flow rate). A further, recently introduced method for the extraction of phytoecdysteroids is the use of deep eutectic solvents and ionic liquids [114], which possess high extraction yields and are fast.
For the screening of large numbers of plant species for the presence of ecdysteroids, the development of micro-extraction methods has been particularly useful as they allow 100–200 samples to be processed by one person in a day. In one method [33], seed samples (ca. 100 mg) were ground in a pestle and mortar before 25–30 mg was transferred to a 1.5 mL Eppendorf vial and extracted 3× with 1 mL MeOH for 3 h at 60 °C. The pooled extracts (3 mL) were mixed with 1.3 mL H2O to give 70% aq. MeOH and then partitioned against 2 × 2 mL hexane to remove non-polar pigments. In a later development of the method [44], the tedious grinding in a pestle and mortar was replaced by weighing the seeds into 2 mL Precellys tubes containing inert ceramic beads and vibrating the tubes to break up the seed material before the extraction with methanol (3 × 1 mL) was performed at 60 °C.
4.2. Partial Purification
Solvent Partitioning
Three solvent partitioning systems have proved particularly useful for the partial purification of phytoecdysteroids, either individually or sequentially. The first is partitioning between aqueous alcohol (50–75% MeOH or EtOH) and hexane or petroleum ether for the removal of non-polar components and pigments. The second is between BuOH and water, where the majority of ecdysteroids partition into the lower BuOH phase. The third is between H2O and CHCl3 or CH2Cl2, where the ecdysteroids partition into the upper water phase. If the plant material is extracted with alcohol, the combined extracts can be modified by the addition of a suitable amount of water, partitioned against hexane. The aq. alcohol phase can be reduced to dryness, dissolved in water, and then partitioned first against CHCl3 and the resulting H2O phase immediately against BuOH, which avoids intermediate rotary evaporation.
4.3. Solid-Phase Extraction
The introduction of Sep-Pak cartridges in the early 1980s revolutionised the partial purification of ecdysteroid-containing samples, initially for relatively small samples but later also for preparative purposes as larger SPE columns became commercially available, and also allowed their simple separation into different classes (polar conjugates, free ecdysteroids, non-polar conjugates) by applying the ecdysteroid-containing sample in 10% MeOH in H2O and sequential step-elution of RP-cartridges with greater percentages of MeOH in H2O [115]. Without a doubt, RP-C18-SPE has become a standard workhorse of ecdysteroid partial purification. A wide range of RP-, NP- and ionic-SPE phases are now available, and these have found niche uses in the purification of specific types of ecdysteroids [107][116].
4.4. Chromatography
For a general review on the chromatography of ecdysteroids, see [107][117].
Thin-Layer Chromatography (TLC)
Although important in the past, the use of TLC in the analysis, quantification and purification of ecdysteroids has now largely been superseded by HPLC. This area is reviewed in [118].
4.5. Open-Column/Flash Chromatography
Open-column chromatography on silica or alumina eluted with solvents of increasing percentages of methanol or ethanol in hexane, dichloromethane or chloroform used to be the standard ‘workhorse’ for the laboratory purification of ecdysteroids, as it was of other plant secondary compounds, but this has gradually been replaced by flash chromatography and preparative HPLC on NP- and RP-stationary phases, which are faster and/or have better resolution [107][117]. Owing to the wide range of polarity of ecdysteroids and ecdysteroid conjugates that may occur in biological samples, it is often desirable to adsorb the sample onto an inert support (e.g., Celite) before application to an NP open- or flash-column since all the components will not dissolve in the non-polar starting elution solvent. Thus, it is often profitable to carry out an initial separation into apolar, intermediate polarity and high polarity ecdysteroids before using preparative HPLC for final purification(s).
4.6. Droplet Counter-Current Chromatography (DCCC)
DCCC, which is a preparative partition system between immiscible stationary and mobile phases, is an effective technique for the purification of ecdysteroids of wide polarity range from complex mixtures [119][120][121] since it is non-destructive and all components elute at some point during the chromatography; it has even been used for an improved purification procedure for the isolation of ecdysone and 20-hydroxyecdysone pupae of the silkworm Bombyx mori [122]. It is not more frequently used because it requires specialised equipment, which is not commonly available, and a separation may take several days.
4.7. Centrifugal Partition Chromatography (CPC)
CPC is a development of DCCC that uses a centrifugal process to speed up the separation process; it is thus fast, efficient and reproducible. It has recently been used in the purification of phytoecdysteroids [123][124].
4.8. High-Performance Liquid Chromatography (HPLC)
The literature on the HPLC separation of all forms of ecdysteroids is very extensive and has been reviewed previously [107][117][125][126][127][128], covering RP and NP chromatography and chromatography on various bonded silicas, the effects of changing the composition of the mobile phase and temperature. The most commonly used HPLC systems are variants on a C18-RP-column eluted with a linear gradient of methanol or acetonitrile in water. This system can be converted to an ion-suppression system by adding buffer or 0.1% formic acid or trifluoroacetic acid, which improves the chromatographic separations of negatively-charged ecdysteroids (ecdysonoic acids, phosphates, sulphates), allowing the full spectrum of ecdysteroids, from polar conjugates through free ecdysteroids to apolar fatty acyl conjugates, to be analysed in one gradient separation.
4.9. Supercritical-Fluid Chromatography (SFC)
SFC can be viewed as a hybrid between GC and NP-HPLC. GC of ecdysteroids is complicated by the polarity of the molecules, necessitating prior (and consistent) derivatization of the multiple hydroxyl groups present in the ecdysteroids. SFC separation of ecdysteroids, using critical CO2, which is non-polar, modified with methanol and silica/bonded (NP) silica HPLC columns or fused silica capillary columns, provides shorter retention times, sharper peaks and greater sensitivity than HPLC. SFC is compatible with UV/vis-, FT-IR- and MS-detection [107][117][129]. Flame-ionisation-detection is also possible if a modifying solvent can be omitted. To this end, it is necessary to reduce the polarity of ecdysteroids, and a convenient derivatisation of 20,22-diol-containing ecdysteroids by the formation of boronic esters has been developed [130].
4.10. Spectral Identification
4.10.1. UV Spectra
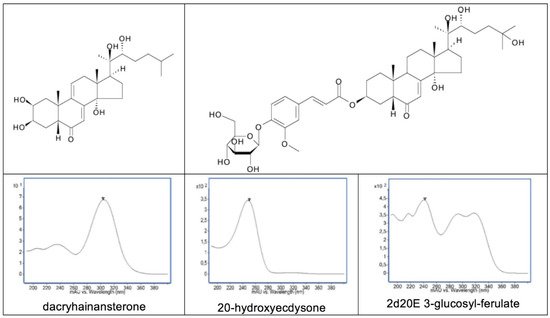
A 14α-hydroxy-Δ7-6-one chromophoric group (as in 20E) is characteristic of most ecdysteroid analogues. This provides a major UV absorbance at 242 nm in MeOH or EtOH (Log ε = 4.103) and a minor absorbance at 310 nm. The maximum wavelength of absorption is shifted slightly depending on the solvent, but it is this absorption that forms the basis for the detection and quantification of ecdysteroids in the vast majority of TLC and HPLC separation systems. In certain ecdysteroid analogues, where the chomophore is modified or extended, the UV spectrum is modified, sometimes extensively. A couple of good examples of this are dacryhainansterone, where the chromphore is extended to a dienone, with a concommittant shift of the maximum absorption to 298 nm (Log ε = 4.152), and the glucopyranosyl ferulate conjugates [109], where the UV spectrum is a combination of the absorptions of the ferulyl group and the characteristic ecdysteroid absorption (Figure 11).
Figure 11. Structures of dacryhainansterone and 2-deoxy-20-hydroxyecdysone 3-glucosylferulate, together with their corresponding UV spectra and that of 20-hydroxyecdysone.
4.10.2. Mass Spectroscopy (MS)
In the early days of the analysis of ecdysteroids by electron-impact MS, it was not easy to obtain the MW owing to the formation of weak [M]+ ions and dehydration of the molecule upon heating of the probe, resulting in a series of losses of 18 amu, depending, in number, on the number of hydroxyl groups present in the ecdysteroid. It was the advent of milder MS techniques (CI, FAB, API) at the beginning of the 1980s that facilitated the analysis of ecdysteroids and, especially, the polar conjugates that were being discovered at that time (e.g., [131]). There followed a period of several years where new MS techniques were developed and assessed for their suitability for the analysis of ecdysteroids (e.g., [132]). The more recent development of dedicated HPLC/DAD/MS equipment has made the analysis, identification and quantification (by MS/MS) of ecdysteroids in complex samples almost routine.
4.10.3. Nuclear Magnetic Resonance Spectroscopy (NMR)
The extensive improvements in the sensitivity, resolution and range of techniques available for NMR over the past 40 years have made it the technique of choice for the identification of any new ecdysteroid analogue since as little as 50 μg of pure compound is often enough to obtain a full structural identification, including the resolution of many of the stereochemical questions, such that it is now normally far easier to solve these by NMR than by X-ray crystallography. Girault and Lafont [133] published the first complete proton-NMR assignments for ecdysone and 20-hydroxyecdysone in 1988. 13C-NMR is also extensively used in the structural identification of ecdysteroids [134][135], as are 1H-1H and 1H-13C two-dimensional techniques [136].
4.11. Hyphenated Techniques
Ecdysteroids and phytoecdysteroid-containing plant extracts have been used as test samples to assess various coupled chromatography/spectroscopy techniques. These have the advantage of providing extra structural information, in addition to the customary retention time and UV/vis spectrum deriving from an HPLC/DAD separation. As the technical problems have been overcome and reliable equipment is now commercially available, HPLC/DAD/MS has become almost routine for the analysis of ecdysteroid mixtures (e.g., [44]). More of a challenge is the direct coupling of an HPLC with a 1H-NMR spectrometer, which has the potential to be a powerful combination, providing considerable structural information concerning the eluting peaks. Two major problems exist for in-line assessment of HPLC effluents by NMR: the customary use of protic solvents to elute HPLC columns and the sensitivity of NMR detection. The former was overcome by using deuterated water in the mobile phase of the RP-HPLC and the latter by using a stopped-flow method to allow adequate time to obtain adequate (if not complete) 1H-spectra in 10 min of stopped-flow with ca. 100 μg of reference ecdysteroids [137]. The coupling has been extended to HPLC/UV/NMR/MS [138][139] by splitting the effluent from the UV detector so that 95% goes to the NMR and 5% to the MS (in-parallel coupling). The chromatography conditions were made more compatible by using deuterated acetonitrile in addition to D2O, and TFA was omitted from the mobile phase as it was found to interfere with the MS. This system could be used with in-flow NMR and MS detection, which allowed the identification of chromatographic peaks in a Silene otites extract that presented strong characteristic 1H-HMR signals and mass spectra, which allowed them to be considered potential ecdysteroids, and then the separation could be repeated in stopped-flow mode to obtain fuller spectra of the peaks of interest.
The scope of the coupling has been extended even further by incorporating an FT-infrared spectrometer in the sequence to give HPLC/DAD/FT-IR/1H-NMR/ToFMS [140] and demonstrating that ecdysteroids can be satisfactorily separated on a C18-RP-HPLC column by superheated (160 °C) D2O alone (i.e., without an organic modifier) [141]. More recently [142], silica HPTLC separation of extracts of Silene spp. has been coupled with desorption electrospray ionization–ion mobility–time-of-flight high-resolution mass-spectrometry (HPTLC/DESI/IM/ToFMS), which permitted the preliminary screening of the plant extracts for ecdysteroids with minimal sample preparation or post-chromatographic processing. Although complicated and expensive to establish, hyphenated techniques have considerable potential in the dereplication of biological samples, i.e., samples can be quite rapidly screened to determine if they contain only already-known analogues or to differentiate the unknown analogues from the known ones so that time and research effort are not wasted in rediscovering compounds [143].
4.12. Immunoassays
Immunoassays allow the sensitive detection of small amounts of analyte in samples of limited purity, so they are suitable for the quantification of levels and the comparison of many samples simultaneously. Thus, they are highly suitable for the determination of hormone levels, such as those of ecdysteroids in arthropods. The ecdysteroids, by themselves, are not immunogenic in mammals as their MWs are normally <1000, so they need to be chemically coupled as hapten groups onto the surface of a suitable protein to raise an immune response. The many hydroxyl groups and the 6-oxo group in ecdysteroids offer several possibilities for linking to a carrier protein, thus allowing various possible orientations of the steroid relative to the surface of the protein, with the consequence that antibodies will preferentially recognise the part of the ecdysteroid molecule that is distal to the linkage site. Each antiserum raised differs in its sensitivity and specificity, and these have to be assessed in each case; the cross-reactivities for a representative range of steroid analogues should be determined relative to the standard ecdysteroid (usually ecdysone or 20-hydroxyecdysone). Consequently, the results for biological samples (which will normally contain a mixture of ecdysteroids in varying proportions) are expressed in equivalents of the standard ecdysteroid (e.g., ng ecdysone equivalents) and the result can arise, at one extreme, from a small amount of highly cross-reactive steroids or, at the other extreme, from a large amount of poorly cross-reactive steroids. Ideally, the immunoassay results should be compared with those obtained for the same samples using other methods (e.g., [144]).
Borst and O’Connor [145] developed the first immunoassay for ecdysteroids, and, over the subsequent years, several others have been developed [146][147][148][149][150][151][152][153][154][155][156][157][158][159]. They differ in their detection principle (radioimmunoassay (RIA; with tritiated or radio-iodinated tracers), enzyme immunoassay (EIA), chemiluminescence immunoassay (CIA)) and their procedural format (separation of “free” from “bound” by using dialysis, ammonium sulphate/ polyethylene glycol/Staphylococcus aureus Protein A precipitation, or charcoal binding), as well as in their sensitivities and specificities.
4.13. Bioassays
The first bioassays for ecdysteroid (or, as it was then known, ‘moulting hormone’) activity were based on the ligated larvae of dipteran flies (Calliphora erythrocephala, Musca domestica, Sarcophaga peregrina) or a lepidopteran (Chilo suppressalis), where the abdomens were isolated from the endogenous source of ecdysteroids (the prothoracic glands) and the abdomens were injected with, or dipped into (in the case of the Chilo test), to determine if pupariation/pupation could be induced [160][161]. These assays were exceedingly important at the time, but they were very time-consuming and only semi-quantitative. The next major development was to use the response of the Drosophila melanogaster Kc cell line to quantify the potency of ecdysteroid analogues [162], which had the advantages of much greater reproducibility and no ecdysteroid metabolism but was still time-consuming as it required the measurement of neurone-like cellular projections that were part of the response. A microplate-based assay using the D. melanogaster BII cell line, where the response could be determined turbidometrically (i.e., directly with a plate reader; [163]), has been used extensively to assess plant extracts for ecdysteroid agonist and antagonist activities and also to quantitatively compare the potencies of purified ecdysteroids and other secondary compounds [37]. The potency of ecdysteroid analogues in this bioassay appears to reflect their binding affinity for the ecdysteroid receptor complex, and the extensive data have been used for QSAR and the development of a pharmacophore hypothesis [164].
5. Sourcing of Large Amounts of Ecdysteroids for Pharmaceutical and Clinical Purposes
Sourcing from Plants
Several approaches could be envisaged to source 20E or other ecdysteroid analogues, but, currently, isolation from an ecdysteroid-accumulating plant species is the only scientifically and financially viable option. Currently, only 20E is commercially available in large amounts, so this will be used as the example in the discussion below:
-
It is perhaps worth reminding ourselves first of the criteria that an ideal plant source should fulfil:
-
The plant should accumulate a high amount of 20E;
-
The plant should have a simple ecdysteroid profile (ideally just 20E);
-
The plant should be easy and rapid to grow in accessible areas of the world;
-
The ecdysteroids should be present in the aerial portions (allowing the roots to regenerate the plant);
-
The plant matrix should be amenable to the ready purification of ecdysteroids;
-
Purification and isolation of 20E should not involve expensive chromatographic methods;
-
The plant should not be susceptible to pests and diseases;
-
The species should not be rare or protected;
-
Culture, harvesting and processing costs should be low; initial processing should take place at the culture site.
Clearly, no plant species will fulfil all these criteria fully, but the closer it comes, the more culturally and commercially viable the challenge of isolating adequate amounts of 20E will become.
Table 1 provides data for the concentrations of 20E in specified parts of selected ecdysteroid-accumulating species. Most ecdysteroid-accumulating species contain between 0.01% and 0.1% of the dry weight as ecdysteroids, whereas the few identified high-accumulators contain 1% and above. The roots of Cyanotis spp. can contain up to 5% of its dry weight as ecdysteroids, largely as 20E [165]. Mature stems of Diploclisia glaucescens were found to contain 3.2% of its dry weight as 20E [166], but the collection of the stems of this South Asian climber precludes this as a feasible source of large amounts of 20E.
The relationship between the presence of phytoecdysteroids and plant taxonomy is complex, but high accumulation is associated with certain species in the genera Achyranthes (Amaranthacea), Cyanotis (Commelinaceae), Pfaffia (Amaranthaceae), Rhaponticum (syn. Leuzea/Stemmacantha; Asteraceae), Serratula (Asteraceae) and Silene (Caryophyllaceae).
The vast majority of currently available commercial sources of 20E are derived from Cyanotis spp., Pfaffia spp., Rhaponticum (Leuzea) spp. or Serratula spp. In addition, turkesterone (11α-hydroxy20E) from Ajuga turkestanica is commercially available.
It has been shown in several ecdysteroid-accumulating species that ecdysteroid levels are additionally influenced by environmental factors (e.g., temperature, nutritional factors, invertebrate predation [35]). Thus, to maximise 20E content in a chosen species, it is not only necessary to select a high producing genetic line (cultivar) but also to optimize the growth conditions and to consider treating the plants with appropriate elicitors (e.g., methyl jasmonate to mimic insect or fungal attack) at suitable times in development.
Various researchers have started to explore a range of culture methods in vitro (e.g., micropropagation, callus cultures, plant cell culture, hairy-root cultures, transformed yeast fermentation) in the hope of obtaining more amenable ecdysteroid-producing systems. To date, only the hairy roots of Ajuga reptans var. atropurpurea [167] consistently and reliably produce ecdysteroids, but the culture is too expensive to provide a commercial source of 20E. Additionally, the hairy roots of some other ecdysteroid-producing species do not contain ecdysteroids, so this cannot be viewed as a general approach. Transforming yeast to biosynthesise ecdysteroids, as has been previously done for certain vertebrate steroids [168][169], is an attractive prospect but is currently confounded by our incomplete knowledge of the ecdysteroid biosynthetic pathways in either invertebrates or plants and the probable number of genes involved in the complete pathway(s) [3].
The major requirements in the processing of plant material to obtain a natural product in adequate amounts for commercial use are simplicity, efficiency of extraction and purification, reproducibility and cost-effectiveness. As explained above, the choice of plant material is key since not only should it contain a large amount of the target molecule, but the plant matrix should be readily extractable and not contain components (e.g., large amounts of polysaccharides) that make processing difficult or significant amounts of close analogues of the target molecule, which would be difficult to separate out without moderate-to-high-resolution chromatographic methods. Given that a suitable plant source will contain typically 1–2% of its dry weight as 20E and that a pharmaceutical/medicinal dose of 20E is likely to be in the 100 mg–2 g/day range, it is necessary to be able to process tonnes of plant material. Clearly, it would be highly advantageous if the harvested plant material could be cleaned, dried, broken up (to reduce volume and increase the surface area) and subjected to initial extraction as close to the site of harvesting as possible, as the mass of the initial extract is probably only 1–2% of the fresh weight of the plant material and, therefore, much more readily and cost-effectively transportable. The extraction and purification have to be optimised with regard to the physico-chemical properties of the target molecule (generally already known or readily determinable) and the nature of the plant matrix (generally only vaguely understood). Owing to the large number of hydroxyl groups in 20E, it is highly soluble in alcohols, so the dried, powdered plant material will usually be extracted with methanol (preferred as it is cheaper) or ethanol (if the product is to be BIO), with or without prior extraction with a non-polar solvent, such as petroleum ether, to de-fat the plant material. Extraction may occur with heating, stirring or maceration for a defined time, all of which need to be optimised. Other methods, such as super critical fluid extraction or bi-phasic extraction, could be used, but these are generally more expensive and would only be cost-effective if the target molecule is potent (low daily dose required) and high-value, which is not the case for 20E.
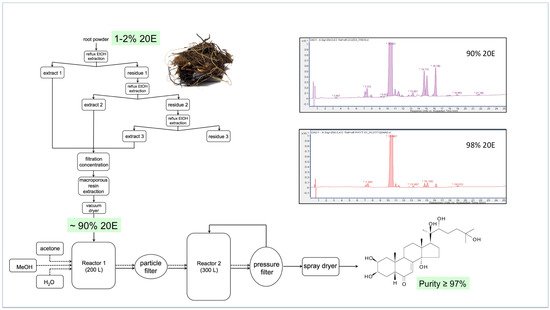
Figure 12 provides a flow diagram of a representative processing method for Cyanotis sp. roots, where the dried plant material is refluxed thrice with ethanol and the pooled extracts are filtered before being passed through a macroporous resin to absorb plant compounds and vacuum-dried to yield a powder containing 90% 20E.
Figure 12. A representative flow diagram for the large-scale extraction and purification of 20E from roots of Cyanotis sp. (taken from [170]).
This preparation can then be recrystallised twice to bring the purity of the 20E to >97%. A comparison of the RP-HPLC profiles of the 90% and 98% 20E preparations is shown in the inset to Figure 10. Most of the minor peaks in the chromatograms correspond to other ecdysteroids, which are very difficult to separate fully from 20E by crystallisation. This underlines the need to start with plant material that contains essentially only 20E.
Owing to the need for optimisation at each stage of the extraction and purification, the process should be developed in stages, going from small-scale (100 g—a few kg of dry plant material) in the laboratory through increasing medium-scale stages (e.g., 10–500 kg), before being applied at the industrial scale (>1 tonne), so that difficulties can be identified and resolved early on and any problems of scale-up can be dealt with. A thorough cost analysis needs to be performed throughout the scale-up procedure to ensure that the target molecule can be brought to market at a viable cost.
For pharmaceutical-grade preparations, the API has to be prepared by a standardised procedure to a defined level of purity (e.g., >97%), and all impurities at levels of >0.5% must be identified, quantified and assessed for toxicity and effects.
6. Alternatives for Large-Scale Production of Ecdysteroids and Their Current Viability
6.1. Chemical Synthesis
Although feasible [171], chemical synthesis of 20-hydroxyecdysone is a complex, multi-step (ca. 18) process with an overall yield of <1%, starting from a precursor that is probably not available at the required scale.
6.2. Cell/Callus Cultures
In vitro cultures from ecdysteroid-rich plants have been developed, but they give disappointing results as the production has been generally very low, even after precursor feeding or hormonal elicitation [172][173][174]. The same applies to callus cultures, indicating that proper tissue organization is a prerequisite for significant ecdysteroid biosynthesis to take place.
6.3. Hairy-Root Cultures
Agrobacterium rhizogenes-transformed roots are induced to proliferate and generate adventitious roots and can produce higher amounts in vitro than those of field-grown plants, but it does not appear so easy to scale up [175] and does not work in all cases [176].
6.4. Plant “Milking”
Chajra et al. [177] have developed a system for inducing the production of secondary metabolites in the roots of plants grown in an aeroponic/hydroponic system and then permeabilising the roots to release their secondary compounds into water. This approach is appropriate for the production of complex, rare and costly metabolites, but large-scale production would require the establishment of a very large, costly infrastructure, and the cost-benefit analysis for 20-hydroxyecdysone is not currently favourable.
6.5. Biofermentation
The use of genetically transformed yeast to produce specific metabolites is raising interest [178]. Whether this can be applied to produce ecdysteroids is, however, a big challenge owing to a complex multi-step process from cholesterol, the fact that it must proceed with a specific sequence involving different cellular compartments and because the early steps (the ‘Black Box’) still require elucidation (see the biosynthetic pathway in insects depicted in Figure 5). Moreover, most of the enzymes, except 14α-hydroxylase [179] and 22-hydroxylase [36], have been identified only in insects/arthropods.
Thus, the only currently feasible approach to the industrial large-scale (tonnes) production of 20-hydroxyecdysone is isolation from a high-accumulating plant source since the alternatives are too inefficient, too costly or exist only in theory.
7. Ecdysteroids: Presence in Human Food and Traditional Medicinal Plants
The presence of ecdysteroids has been investigated in food plants, and it has been detected in a few species only [43][44][180], e.g., spinach [181], quinoa [182], yam tubers [183] and in some mushrooms [32][42]. We may wonder why the presence of ecdysteroids is so limited in cultivated species. It is possible that during the domestication process, varieties were selected based on their productivity, and thus the synthesis of secondary metabolites was counter-selected as their synthesis is energy-consuming for the plant. The presence of significant ecdysteroid concentrations in cultivated Chenopodiaceae (quinoa, spinach) seems to represent an exception to this rule, and eating those plants can provide up to 20–40 mg of 20E per portion, which is not negligible.
As the phytochemical analysis of traditional medicinal plants from around the world has advanced, it has become apparent that some of them may contain large amounts of ecdysteroids (Table 1 in [170]). This interesting association may indicate that ecdysteroids, alone or in conjunction with other plant secondary compounds (e.g., flavonoids), may have very wide therapeutic applications, but it must be borne in mind that ecdysteroids have not been proven to be responsible for all the pharmacological activities of these plant extracts.
Based on the traditional use of some of these plants, dried plant parts (or, rather, their extracts) are marketed as dietary supplements (Figure 13), mainly promoting their anabolic properties (in humans (bodybuilders, sportsmen), but also in animals (horses, dogs))—see [184] for a list of available products already available 18 years ago.
| Hematopoiesis stimulation |
| Bone and cartilage protective |
| Angiogenic |
| Skin protective/repairing |
The mechanism of action of 20E is not yet fully understood [170][192]. Given the pleiotropy of its effects, it clearly interacts with receptors expressed in many tissues. Parr et al. [193] established the involvement of estrogen (nuclear) receptor-β, whereas Gorelick-Feldman et al. [194] provided pharmacological evidence for 20E action through a membrane GPCR receptor. Recently, Lafont et al. [192] proposed a unifying mechanism involving both the GPCR Mas (a membrane receptor of angiotensin-(1–7)) and a membrane-bound estrogen receptor, but this still deserves further confirmation.
As 20-hydroxyecdysone became available in large (kg) amounts, its practical use developed in two areas: (i) external use in cosmetics, aimed at improving skin quality/repair (e.g., [195][196][197][198][199][200]), and (ii) internal use, with a wide array of ecdysteroid-containing nutritional supplements for humans (bodybuilders, sportsmen) and also horses and dogs (see above). However, this seems very restrictive with regard to the multiple beneficial effects already observed with animal models.
The muscle anabolic properties have been investigated in small-scale human trials over the past 35 years (Table 4), in particular the earlier ones that used the standardized proprietary preparation Ecdysten®, pills, containing 5 mg of pure 20E extracted from Rhaponticum carthamoides. For most of them, no detailed reports are available. The administration of the molecules was often combined with protein supplements (e.g., whey proteins) and intense physical exercise, which makes it difficult to determine the effects of the ecdysteroids themselves.
Table 4. Human studies on anabolic properties of ecdysteroids.
| Aim |
Age |
N |
Dose |
Duration |
Output |
Reference |
| Physical capacity |
Athletes 18–28 |
117 |
? |
? |
Ecdysterone treatment improves oxygenation and decreases recovery time after exercise |
[201] |
| Anabolic effect |
Young adults |
10 M, 10 F /arm |
25 mg 3 x/day |
10 days |
Ecdysten and/or protein supplement + physical training evoke a reduction of fat mass (−7% M, −14% F) and an increase in muscle mass (+6% M, 7% F) |
[202] |
| Anabolic effect |
Runners 15–25 |
20 (4 arms) |
? |
21 days |
Reduction of subcutaneous fat, muscle mass increase |
[203] |
| Physical capacity |
Athletes |
44 |
? |
20 days |
Increase of working capacity by 10–15% |
[204] |
| Endurance |
Athletes |
?? |
3 weeks |
A combination of ecdysten and cytamins increases bench press performance and endurance |
[206] |
| Physical capacity |
20.5 ± 3 |
45 M |
30 mg/day * |
8 weeks |
No observed effects on body composition, anabolic/catabolic hormonal status, or physical performance |
[207] |
| Physical capacity |
Athletes |
64 M |
5, 10, then 15 mg/day |
10 days each |
Muscle mass increase (+5%), fat mass decrease, strength increase (+12%) |
[208] |
| Anabolic effect |
Athletes 25.6 ± 3.7 |
46 |
12/48 mg/day ** |
10 weeks |
[170]. They are also currently being assessed in a clinical trial on SARS-CoV-2 patients with regard to their lung-protective activity [211].
9. Conclusions
-
Ecdysteroids are widespread in nature. They are the steroid hormones of arthropods and other invertebrates and are, in view of the predominance of these animals in the world, the most frequently occurring steroid hormones, even if vertebrate steroid hormones receive more scientific attention. They also occur in plants, where their occurrence is not universal (although the genetic capacity to produce them may be), but when they do occur, they can accumulate to high concentrations.
-
The analysis, identification and quantification of ecdysteroids are highly advanced, and new analogues (particularly in plants) are continuing to be identified. Perhaps uniquely, the data and literature concerning ecdysteroids have been compiled, are readily available online and are continuously updated (www.ecdybase.com).
| ? |
| Enhancement of endurance performances and of immune system response |
| [ |
| 205 | ] |
| Increase in body weight (ca. 3 kg), muscle hypertrophy, and improved performance at bench press |
| [ | 209 | ] |
In any case, the association of ecdysteroids, protein-enriched diet and exercise seems effective on young people to promote muscle gain (and fat loss). Ecdysteroids are currently being evaluated by WADA to possibly include them in the list of prohibited doping agents to be banned for sportsmen [210].
On the other hand, ecdysteroids have been or are being presently investigated in clinical trials in order to assess their potential use as a medicine for treating parasitoses or several diseases, and this list is not closed
-
The mode of action of ecdysteroids in some model arthropod species is now fairly well understood, but, in view of the enormous diversity of arthropods, much certainly remains to be discovered. The control of development by ecdysteroids in other invertebrates is still poorly understood.
-
Phytoecdysteroids appear to possess allelochemical functions in the plants where they occur by acting as either antifeedants or endocrine disruptors to invertebrate predators. The wide diversity of phytoecdysteroid analogues would appear to be a consequence of this competition for survival between ecdysteroid-containing plants and the behavioural and biochemical strategies of invertebrate predator species to cope with this class of molecules.
-
Ecdysteroids have many positive pharmacological effects on mammals. The modes of action by which these effects occur are now starting to be understood. This aspect of ecdysteroid research is currently the one with the greatest potential, as indicated by the steady rise in the number of clinical trials being performed to assess the medical uses of ecdysteroids.
10. Prospects and Applications
-
The biosynthesis of ecdysteroids in arthropods and plants remains to be fully elucidated. Final clarification of the biochemical pathways, enzymology and regulation remains a major challenge, which would considerably facilitate many strategies for the agricultural and medical applications of ecdysteroids.
-
Although high concentrations of exogenous ecdysteroids are generally deleterious to invertebrate development, it is clear that low doses can have beneficial developmental and commercial consequences for the rearing of silkworms, bees, prawns and crabs. This area can be expected to develop considerably into established cottage industries as our knowledge of local ecdysteroid-containing plants grows, fueled by the general commercial availability of purified 20E at a reasonable cost.
-
Certain plant species are high accumulators of ecdysteroids (mainly 20E) and are thus very good commercial sources of these compounds, especially in the current absence of other viable strategies to obtain ecdysteroids. The availability of reliable, pure preparations of ecdysteroids is essential for continuing clinical trials and for the ultimate commercialisation of medical drugs with ecdysteroids as their API.
-
The current emphasis is on medical applications that concern the potential anabolic effects of ecdysteroids, especially those concerning muscle wasting (e.g., sarcopenia, Duchenne muscular dystrophy). Such research will continue apace, but it is to be expected that other medical conditions could benefit from other beneficial pharmacological effects of ecdysteroids (see Table 3).
-
Many nutritional supplements that claim that they contain specified analogues at specified levels are commercially available. However, quantitative and qualitative analyses demonstrate that very few of these contain the stated analogue (20E or turkesterone) at the required level; they may contain other unstated analogues. Further, ecdysteroid contents and profiles can vary from batch to batch. This area needs regulation as purchasers are not receiving what they thought; there is no information on the identity or safety of other plant components present and these poor and variable preparations could result in ecdysteroids unfairly receiving a bad reputation.
-
Evidence is beginning to accumulate that the pharmacological effects are not specific to ecdysteroids but may be a more general property of polyhydroxylated steroids (as proposed by Karel Sláma in 1993 [212]) since anabolic, hypoglycaemic and wound-healing effects are also associated with brassinosteroids in rodents [213][214][215] and withaferin A (withanolide A; Figure 15) protects against high-fat-induced obesity in mice [216]. This lack of absolute specificity may explain why relatively high concentrations of polyhydroxylated steroids are required for activity. If this is substantiated, it is probable that 20E would be the polyhydroxylated steroid of choice because of its commercial availability in large amounts and its demonstrated lack of toxicity in mammals.
Figure 15. Structures of 28-homobrassinolide (A) and withaferin A (B).