You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Encyclopedia:
From Scholars for Scholars
Trending Entry
News and Events
More >>
Announcement
16 Apr 2025
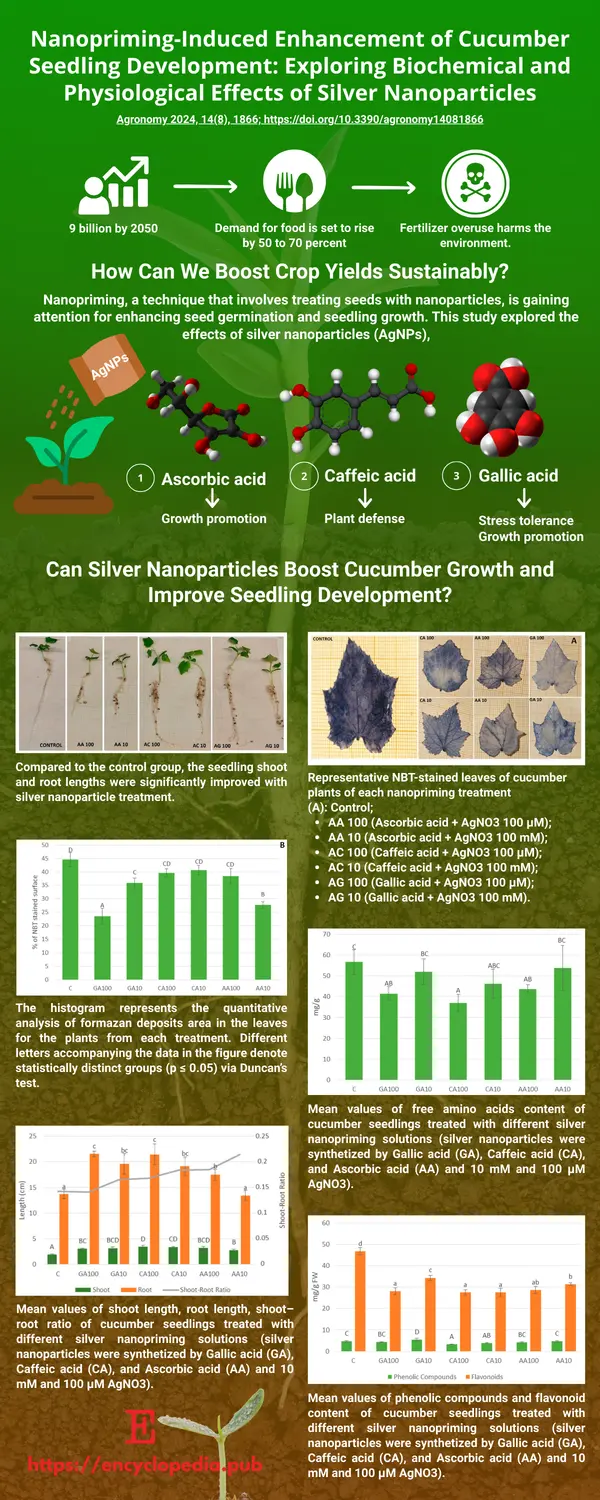
Nanopriming, the process of treating seeds with nanoparticles, is emerging as a promising strategy to improve seed germination and early plant growth. In a recent study, researchers investigated the impact of silver nanoparticles (AgNPs) on cucumber seedlings. These nanoparticles were synthesized using natural reducing agents—ascorbic acid, caffeic acid, and gallic acid—resulting in spherical particles with distinct optical properties.
Source: https://encyclopedia.pub/image/3390
The findings revealed that AgNP treatments generally led to improved germination rates and enhanced shoot and root growth compared to untreated seeds. However, the effects varied depending on the type and concentration of the reducing agent used in the synthesis.
Biochemical analyses showed that AgNP exposure influenced several physiological markers. Changes were observed in reactive oxygen species (ROS) levels, oxidative stress indicators, and concentrations of amino acids, phenolic compounds, flavonoids, and soluble sugars. Notably, some treatments helped reduce oxidative stress, while others increased oxidative damage, indicating that the response to AgNPs is complex and formulation-dependent.
This study highlights the potential of nanopriming as an agricultural tool and points to the need for further research to fine-tune nanoparticle formulations for specific plant species and desired outcomes.
For more information about the research, you can view the online video entitled "Nanopriming-Induced Enhancement of Cucumber Seedling Development".
Ongoing
07 Apr 2025
Encyclopedia MDPI is thrilled to announce significant enhancements to its Academic Video Service, which aim to improve its quality, accessibility, and functionality. Since its launch, our video service has enabled numerous scholars to present their research in a dynamic and visually engaging format, greatly enhancing its visibility and impact.
Due to the overwhelmingly positive reception this service has received, we have reached a point where the number of orders we are receiving exceeds our current capacity. In order to maintain the quality of these videos and continue optimizing the service, we have made the decision to introduce a fee. However, to ensure that this service is still a cost-effective option, we have set our prices significantly below the market average.
Highlights of the Upgrades to the Service
Although the service will now be fee-based, we are committed to providing even more professional and comprehensive support, including the following:
One-on-one video production guidance
Personalized assistance to ensure your needs are fully met.
Scriptwriting and English editing
Expertly crafted narratives and professional English editing to ensure your research is presented clearly, accurately, and with impact.
High-quality animations
Visually engaging animations are created to simplify complex research and captivate your audience.
Whiteboard Animations: Clean and minimalist, using hand-drawn illustrations to explain ideas step-by-step.
Motion Graphics (MG) Animations:
Cartoon Style: Bright, colorful, and approachable, ideal for making technical or scientific content more accessible and engaging.
Hand-Drawn Style: Unique and artistic, adding a personal touch to your research while maintaining clarity and professionalism.
Customized infographics (optional)
We can also create tailored infographics to visually summarize key data or findings, enhancing the clarity and appeal of your video.
Native voiceover
Native speakers provide voiceovers to enhance the accessibility and reach of your research.
Multiple rounds of revision
To ensure your video accurately represents your work.
Social media promotion
Expanding your research's visibility and impact.
Why Choose Us?
The Proven Impact of Video Abstracts
Research shows that a well-crafted video abstract can significantly enhance the visibility and impact of your work. It has been shown to do the following:
Increase paper views by 120% (Source: 10.1007/ s11192-019-03108-w)
Boost citations by 20% (Source: Wiley Online Library)
Improve journal rankings by 33% (Source: Research Square)
Raise Altmetrics scores by 140% (Source: Research Square)
Our Expertise in Academic Research
Backed by MDPI, our experienced production team combines deep academic knowledge with creative excellence. We understand the nuances of scholarly communication and ensure that every frame accurately conveys the value of your research, meeting the highest standards of quality and precision.
Collaborations with SCI Journals
We have partnered with many SCI journals to create exclusive video series, enhancing the dissemination and impact of published research. For example, our collaborations with Entropy, Remote Sensing, Nanomaterials , Animals , Nutrients, Foods , Sustainability, Encyclopedia, Cancers, etc., have helped authors achieve greater visibility and recognition for their work.
Global visibility
The videos are linked to your paper's DOI for maximum exposure.
Available Video Services and Their Pricing
Video Abstract (up to 5 minutes long): Summarizes the key findings, methodology, and significance of your research paper.
Regular price: CHF 600
Discounted Price: CHF 400
Short Take (up to 2 minutes long): Uses original animations to explain the specific aspects of your research.
Regular price: CHF 500
Discounted Price: CHF 300
Scholar Interview: A face-to-face discussion offering deeper insights into your publication.
Regular price: CHF 400
Discounted Price: CHF 200
Special Offer
To express our gratitude for your support, we are pleased to offer a buy one get one free promotion. This offer is valid for two weeks, and the complimentary service provided can be utilized within one year.
Video Production Service
If you want to see some examples of our videos, please visit https://encyclopedia.pub/video.
If you would like to apply for the video service, please click https://encyclopedia.pub/video_service.
Others
If you have any other questions, please contact office@encyclopedia.pub.
Announcement
16 Apr 2025
Nanopriming, the process of treating seeds with nanoparticles, is emerging as a promising strategy to improve seed germination and early plant growth. In a recent study, researchers investigated the impact of silver nanoparticles (AgNPs) on cucumber seedlings. These nanoparticles were synthesized using natural reducing agents—ascorbic acid, caffeic acid, and gallic acid—resulting in spherical particles with distinct optical properties.
Source: https://encyclopedia.pub/image/3390
The findings revealed that AgNP treatments generally led to improved germination rates and enhanced shoot and root growth compared to untreated seeds. However, the effects varied depending on the type and concentration of the reducing agent used in the synthesis.
Biochemical analyses showed that AgNP exposure influenced several physiological markers. Changes were observed in reactive oxygen species (ROS) levels, oxidative stress indicators, and concentrations of amino acids, phenolic compounds, flavonoids, and soluble sugars. Notably, some treatments helped reduce oxidative stress, while others increased oxidative damage, indicating that the response to AgNPs is complex and formulation-dependent.
This study highlights the potential of nanopriming as an agricultural tool and points to the need for further research to fine-tune nanoparticle formulations for specific plant species and desired outcomes.
For more information about the research, you can view the online video entitled "Nanopriming-Induced Enhancement of Cucumber Seedling Development".
Ongoing
07 Apr 2025
Encyclopedia MDPI is thrilled to announce significant enhancements to its Academic Video Service, which aim to improve its quality, accessibility, and functionality. Since its launch, our video service has enabled numerous scholars to present their research in a dynamic and visually engaging format, greatly enhancing its visibility and impact.
Due to the overwhelmingly positive reception this service has received, we have reached a point where the number of orders we are receiving exceeds our current capacity. In order to maintain the quality of these videos and continue optimizing the service, we have made the decision to introduce a fee. However, to ensure that this service is still a cost-effective option, we have set our prices significantly below the market average.
Highlights of the Upgrades to the Service
Although the service will now be fee-based, we are committed to providing even more professional and comprehensive support, including the following:
One-on-one video production guidance
Personalized assistance to ensure your needs are fully met.
Scriptwriting and English editing
Expertly crafted narratives and professional English editing to ensure your research is presented clearly, accurately, and with impact.
High-quality animations
Visually engaging animations are created to simplify complex research and captivate your audience.
Whiteboard Animations: Clean and minimalist, using hand-drawn illustrations to explain ideas step-by-step.
Motion Graphics (MG) Animations:
Cartoon Style: Bright, colorful, and approachable, ideal for making technical or scientific content more accessible and engaging.
Hand-Drawn Style: Unique and artistic, adding a personal touch to your research while maintaining clarity and professionalism.
Customized infographics (optional)
We can also create tailored infographics to visually summarize key data or findings, enhancing the clarity and appeal of your video.
Native voiceover
Native speakers provide voiceovers to enhance the accessibility and reach of your research.
Multiple rounds of revision
To ensure your video accurately represents your work.
Social media promotion
Expanding your research's visibility and impact.
Why Choose Us?
The Proven Impact of Video Abstracts
Research shows that a well-crafted video abstract can significantly enhance the visibility and impact of your work. It has been shown to do the following:
Increase paper views by 120% (Source: 10.1007/ s11192-019-03108-w)
Boost citations by 20% (Source: Wiley Online Library)
Improve journal rankings by 33% (Source: Research Square)
Raise Altmetrics scores by 140% (Source: Research Square)
Our Expertise in Academic Research
Backed by MDPI, our experienced production team combines deep academic knowledge with creative excellence. We understand the nuances of scholarly communication and ensure that every frame accurately conveys the value of your research, meeting the highest standards of quality and precision.
Collaborations with SCI Journals
We have partnered with many SCI journals to create exclusive video series, enhancing the dissemination and impact of published research. For example, our collaborations with Entropy, Remote Sensing, Nanomaterials , Animals , Nutrients, Foods , Sustainability, Encyclopedia, Cancers, etc., have helped authors achieve greater visibility and recognition for their work.
Global visibility
The videos are linked to your paper's DOI for maximum exposure.
Available Video Services and Their Pricing
Video Abstract (up to 5 minutes long): Summarizes the key findings, methodology, and significance of your research paper.
Regular price: CHF 600
Discounted Price: CHF 400
Short Take (up to 2 minutes long): Uses original animations to explain the specific aspects of your research.
Regular price: CHF 500
Discounted Price: CHF 300
Scholar Interview: A face-to-face discussion offering deeper insights into your publication.
Regular price: CHF 400
Discounted Price: CHF 200
Special Offer
To express our gratitude for your support, we are pleased to offer a buy one get one free promotion. This offer is valid for two weeks, and the complimentary service provided can be utilized within one year.
Video Production Service
If you want to see some examples of our videos, please visit https://encyclopedia.pub/video.
If you would like to apply for the video service, please click https://encyclopedia.pub/video_service.
Others
If you have any other questions, please contact office@encyclopedia.pub.
Announcement
16 Apr 2025
Nanopriming, the process of treating seeds with nanoparticles, is emerging as a promising strategy to improve seed germination and early plant growth. In a recent study, researchers investigated the impact of silver nanoparticles (AgNPs) on cucumber seedlings. These nanoparticles were synthesized using natural reducing agents—ascorbic acid, caffeic acid, and gallic acid—resulting in spherical particles with distinct optical properties.
Source: https://encyclopedia.pub/image/3390
The findings revealed that AgNP treatments generally led to improved germination rates and enhanced shoot and root growth compared to untreated seeds. However, the effects varied depending on the type and concentration of the reducing agent used in the synthesis.
Biochemical analyses showed that AgNP exposure influenced several physiological markers. Changes were observed in reactive oxygen species (ROS) levels, oxidative stress indicators, and concentrations of amino acids, phenolic compounds, flavonoids, and soluble sugars. Notably, some treatments helped reduce oxidative stress, while others increased oxidative damage, indicating that the response to AgNPs is complex and formulation-dependent.
This study highlights the potential of nanopriming as an agricultural tool and points to the need for further research to fine-tune nanoparticle formulations for specific plant species and desired outcomes.
For more information about the research, you can view the online video entitled "Nanopriming-Induced Enhancement of Cucumber Seedling Development".
Featured Images
More >>
Encyclopedia Editorial Office
- 15 Apr 2025
Encyclopedia Editorial Office
- 26 Mar 2025
Journal Encyclopedia - Peer-Reviewed Content
More >>
Encyclopedia 2025, 5(2), 43; https://doi.org/10.3390/encyclopedia5020043
Peer Reviewed
Encyclopedia 2025, 5(2), 44; https://doi.org/10.3390/encyclopedia5020044
Peer Reviewed
Encyclopedia 2025, 5(2), 45; https://doi.org/10.3390/encyclopedia5020045
Encyclopedia 2025, 5(2), 43; https://doi.org/10.3390/encyclopedia5020043
Peer Reviewed
Encyclopedia 2025, 5(2), 44; https://doi.org/10.3390/encyclopedia5020044
Peer Reviewed
Encyclopedia 2025, 5(2), 45; https://doi.org/10.3390/encyclopedia5020045
Encyclopedia 2025, 5(2), 43; https://doi.org/10.3390/encyclopedia5020043
Peer Reviewed
Encyclopedia 2025, 5(2), 44; https://doi.org/10.3390/encyclopedia5020044
See what people are saying about us
Sandro Serpa
With the precious and indispensable active participation of scholars from all over the world, in articulation with the Editorial Office Team, will certainly continue to give a positive response to the new challenges emerging.
Department of Sociology, Faculty of Social and Human Sciences, University of the Azores, Portugal
Hsiang-Ning Luk
I received a feedback from a particular reader and now we intend to collaborate on a paper together. I appreciate the MDPI provides such a good platform to make it happen.
Department of Anesthesia, Hualien Tzu-Chi Hospital, Hualien, Taiwan
Melvin R. Pete Hayden
Thank the video production crew for making such a wonderful video. The narrations have been significantly added to the video! Congratulations on such an outstanding job of Encyclopedia Video team.
University of Missouri School of Medicine, United States
Shlomi Agmon
Encyclopedia Video provides potential readers with a tool to quickly understand what the work is about. That is important for casualreaders, whose time is thus spared, and for investedreaders, for whom it makes the decision to say "yes, I want to read the paper" much simpler.
School of Computer Science and Engineering, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel
Ignacio Cea
For the video abstracts, the papers and authors could gain more visibility and increase citations. Also, it means a more diverse and interesting way of communicating research, which is something valuable in itself.
Center for Research, Innovation and Creation, and Faculty of Religious Sciences and Philosophy, Temuco Catholic University
Patricia Jovičević-Klug
I am really impressed by the final video (produced by Encyclopedia Video team). I was a little bit worried about what the final video would be like, because of the topic, but you made the magic come true again with this video.
Department of Interface Chemistry and Surface Engineering, Max-Planck-Institute for Iron Research, Max-Planck-Str. 1, 40237 Düsseldorf, Germany
Bjørn Grinde
I believe research should be brought to the public and that videos are important for getting there. A warm thanks to Encyclopedia for making it happen and to the video crew for doing an excellent job.
Division of Physical and Mental Health, Norwegian Institute of Public Health, Oslo, Norway
Patricia Takako Endo
I have followed up the Encyclopedia initiative and I think it is a good alternative where researchers can share their works and results with the community.
Programa de Pós-Graduação em Engenharia da Computação Pernambuco, Universidade de Pernambuco, Brazil
Sandro Serpa
With the precious and indispensable active participation of scholars from all over the world, in articulation with the Editorial Office Team, will certainly continue to give a positive response to the new challenges emerging.
Department of Sociology, Faculty of Social and Human Sciences, University of the Azores, Portugal
Hsiang-Ning Luk
I received a feedback from a particular reader and now we intend to collaborate on a paper together. I appreciate the MDPI provides such a good platform to make it happen.
Department of Anesthesia, Hualien Tzu-Chi Hospital, Hualien, Taiwan
Melvin R. Pete Hayden
Thank the video production crew for making such a wonderful video. The narrations have been significantly added to the video! Congratulations on such an outstanding job of Encyclopedia Video team.
University of Missouri School of Medicine, United States
Shlomi Agmon
Encyclopedia Video provides potential readers with a tool to quickly understand what the work is about. That is important for casualreaders, whose time is thus spared, and for investedreaders, for whom it makes the decision to say "yes, I want to read the paper" much simpler.
School of Computer Science and Engineering, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel
Ignacio Cea
For the video abstracts, the papers and authors could gain more visibility and increase citations. Also, it means a more diverse and interesting way of communicating research, which is something valuable in itself.
Center for Research, Innovation and Creation, and Faculty of Religious Sciences and Philosophy, Temuco Catholic University
Patricia Jovičević-Klug
I am really impressed by the final video (produced by Encyclopedia Video team). I was a little bit worried about what the final video would be like, because of the topic, but you made the magic come true again with this video.
Department of Interface Chemistry and Surface Engineering, Max-Planck-Institute for Iron Research, Max-Planck-Str. 1, 40237 Düsseldorf, Germany
Bjørn Grinde
I believe research should be brought to the public and that videos are important for getting there. A warm thanks to Encyclopedia for making it happen and to the video crew for doing an excellent job.
Division of Physical and Mental Health, Norwegian Institute of Public Health, Oslo, Norway
Patricia Takako Endo
I have followed up the Encyclopedia initiative and I think it is a good alternative where researchers can share their works and results with the community.
Programa de Pós-Graduação em Engenharia da Computação Pernambuco, Universidade de Pernambuco, Brazil
Sandro Serpa
With the precious and indispensable active participation of scholars from all over the world, in articulation with the Editorial Office Team, will certainly continue to give a positive response to the new challenges emerging.
Department of Sociology, Faculty of Social and Human Sciences, University of the Azores, Portugal
Hsiang-Ning Luk
I received a feedback from a particular reader and now we intend to collaborate on a paper together. I appreciate the MDPI provides such a good platform to make it happen.
Department of Anesthesia, Hualien Tzu-Chi Hospital, Hualien, Taiwan
Melvin R. Pete Hayden
Thank the video production crew for making such a wonderful video. The narrations have been significantly added to the video! Congratulations on such an outstanding job of Encyclopedia Video team.
University of Missouri School of Medicine, United States