Intersphincteric resection is an anus-preserving technique for low rectal cancers described by Schiessel et al. in 1994 as a combination of two techniques: the intersphincteric rectal excision for inflammatory bowel disease and the coloanal anastomosis for low rectal resections.
1. Intersphincteric resection (ISR)
Intersphincteric resection (ISR) is characterized by two distinct phases: abdominal and perineal. ISR allows extension of the caudal dissection plane to allow a safe distal margin for very low-lying rectal cancer without excising the sphincter complex (external anal sphincter (EAS)/levator ani muscle (LAM)) as in the abdominoperineal resection. The oncological safety of the ISR derives from the knowledge that lymphatic spread of low rectal cancers occurs especially in the oral direction within the mesorectum with local spread present only in few millimeters [1][2].
ISR was originally classified as subtotal and total according to the partial or complete resection of the IAS
[3]. However, the Japanese experience on ISR has modified the original classification into three types (
Figure 1)
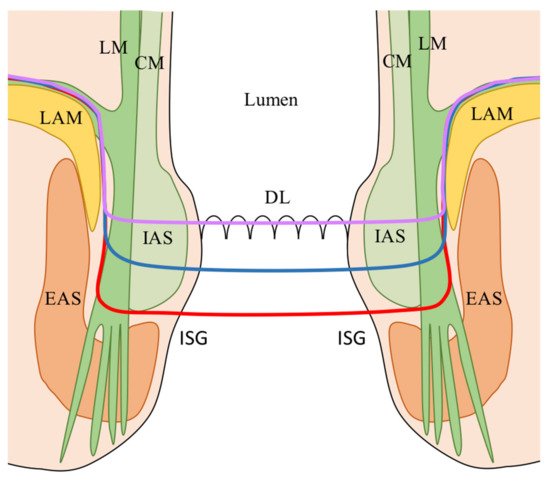
[4][5]: (1) Total ISR (complete removal of the internal anal sphincter at the intersphincteric groove); (2) Subtotal ISR (the resection line lays between the dentate line (DL) and the intersphincteric groove (ISG); (3) Partial ISR (the resection is at the level of the DL. The choice of the dissection line depends on the lower border of the tumor in order to obtain an adequate distal clearance (distal resection margin (DRM) ≥ 1 cm).
Figure 1. ISR classification according to the extension of dissection
[4][5]. Red line: total ISR (complete resection of the IAS at the (ISG); Blue line: subtotal ISR (resection line between the DL and the (ISG); Purple line: partial ISR (resection at the level of the (DL). CM: Circular muscle of the anal canal; DL: Dentate line; EAS: External anal sphincter; IAS: Internal anal sphincter; ISG: Intersphincteric groove; LAM: levator ani muscle; LM: Longitudinal muscle of the anal canal.
The intersphincteric dissection for ISR is usually started in the transabdominal phase and completed during the perineal phase
[6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21]. However, some authors perform the intersphincteric dissection in toto only during the perineal phase
[22][23][24][25][26][27][28][29][30]. Park et al. and Kim et al. have reported the use of the robotic platform to perform total/near-total single-stage transabdominal ISR with no need for a perineal intersphincteric dissection
[31][32].
2. Indication of ISR
ISR is a surgical technique for treating patients with LRC, generally defined as tumors with the caudal edge within 4–5 cm from the anal verge (AV) or 2 cm above the DL. Surgical indications for ISR have changed since the standardization of the technique
[3] (
Table 1). However, a precise preoperative staging with the combination of rectal MRI, thoracic-abdominopelvic computed tomography (CT) scan, anal EUS, rigid proctoscopy, and digital rectal examination (DRE) remains crucial for a correct surgical indication
[33]. Restaging should be done after neoadjuvant chemoradiation (nCRT), and surgical indications must be always re-checked and re-discussed with the patient. The final decision to perform an ISR or convert to APR is done by the surgeon in the operating theatre, before starting surgery, while performing DRE under anesthesia to access tumor mobility and relationship to the anal sphincters
[13][24].
The reviews of Martin et al.
[33], Akagi et al.
[4], and Shirouzu et al.
[34] have critically discussed the indication criteria for ISR. They all agreed that ISR should be indicated for well/moderately differentiated T1–3 tumors located within 5 cm from the AV, independently to IAS invasion. Poorly differentiated, T4, fixed tumors (at DRE), with EAS/LAM infiltration and/or untreatable distant metastases should be contraindicated to ISR. Moreover, ISR should not be indicated to patients with poor anal function, severe preoperative pathologies (cardiac failure, liver cirrhosis, renal dysfunction, and respiratory dysfunction), or psychiatric disease. These indications were confirmed by a national-based questionnaire from the Japanese Society for Cancer of the Colon and Rectum (JSCCR)
[35].
Rullier et al. implemented the indication criteria for LRC, through an MRI-based classification
[36]. They classified LRC into four types to assist decision-making between anus-preserving surgery and APR. Type I are LRC defined as supra-anal tumors (lesions >1 cm from the anorectal ring). Type II are defined as juxta-anal tumors (lesions ≤ 1 cm from the anorectal ring). Type III are defined as intra-anal tumors (lesions with IAS invasion). Type IV are defined as transanal tumors (lesions with EAS or LAM invasion). They indicated ISR for type II and III. Furthermore, some authors have proposed to combine ISR with partial resection of the EAS for type IV tumors
[5][37][38]. Rullier’s classification is very intuitive and of easy use, however, it evaluates the tumor position, from MRI images, in relation to the LAM and EAS in a frontal view without considering the circumferential position of the tumor on the anal clock. Through a retrospective analysis of surgical specimens, Kang et al. analyzed the circumferential tumor location, reporting that the anterior aspect most frequently involves the circumferential resection margin (CRM) and exhibits a deeper tumor invasion
[39]. Further studies are needed to define if the circumferential tumor location may in the future play a role in the treatment strategy, such as stronger indication for preoperative radiotherapy or choice of surgical approach.
The indication criteria for ISR have been recently discussed by two studies. Park et al.
[20] have examined the role of the tumor’s response to nCRT on restaging pelvic MRI as indication criteria for ISR. They reported the ymrT stage and ymrCRM status as key factors for deciding between ISR and APR. Moreover, ISR indication was extended also to patients with cT4 LRS that downstaged after CRT (i.e., ymrT0–3). Poor responders (i.e., ymrT3) with suspicious tumor invasion of the CRM should instead undergo APR.
Piozzi et al. reported a study on 161 ISR where indication criteria were extended. In this study patients with post nCRT clearance of EAS/LAM infiltration were indicated to ISR independently to T stage if curative resection was considered technically feasible at the pre-operative MRI staging
[21].
Clinical indications to ISR have changed in the last three decades however an international consensus should be taken to critically define them in order to perform standardized ISR with comparable results throughout the colorectal community.
Table 1. Indications for ISR according to the literature.
| Authors, Year |
Indications |
Contraindications |
| Schiessel, 1994–2012 [3][6][1] |
-T1–T3 LRC
-Tumor diameter >1 cm
-Big villous adenomas
-Mucosectomy/RT residual tumors
-Low carcinoids/hemangiomas |
-Undifferentiated tumors
-EAS infiltration
-T4 stage
-Preoperative insufficient sphincter function
-Distant metastases |
| Vorobiev, 2004 [22] |
T2–3 (EUS)
Well/moderately diff. adenoca.
Fecal continence |
-EAS/LAM infiltration
-N+ (EUS)
-M+ |
| Rullier, 2005 [9] |
-≤4.5 cm AV
-Distant metastases |
-EAS/LAM infiltration
-Fixed tumors (except partial vaginal fixity)
-Fecal incontinence > 6 months before diagnosis |
| Hohenberger, 2005 [10] |
-≥0.5 cm from DL (rectoscopy)
-T1–2 (EUS)
-T3 (above puborectal sling)
-G1–2
-Patients with possibly distinct invasion of the pelvic floor musculature underwent prior nCRT |
-EAS infiltration
-Fecal incontinence |
| Chin, 2006 [11] |
-T2
-T3–4 (after nCRT)
-≤5 cm (maximal diameter)
-1–3 cm from DL |
-Distant metastases |
| Chamlou, 2007 [12] |
-T1–3
-T4 if invasion is distant from the tumor’s lowest part/sphincter, and is resectable
-Resectable distant metastases
-uT1 with adverse pathologic features after transanal local excision |
-EAS/LAM infiltration
-Fecal incontinence |
| Krand, 2009 [23] |
-(Study on ISR with partial IAS)
-Distal excision at the DL or 1–2 mm distal to it
-T2–3
-Well/moderately diff. adenoca. |
-Total IAS for achieving acceptable DRM
-Fecal incontinence
-EAS/LAM infiltration
-Poorly diff. adenoca.
-Distant metastases (except resectable liver metastases) |
| Han, 2009 [24] |
-T1–2 (IAS)
-T1-T2 after nCRT
-Tumor diameter > 1 cm but <5 cm
-Well/moderately diff. adenoca.
-Sufficient anal function (DRE, manometry) |
-Infiltration of pelvic floor
-Tumor diameter > 5 cm
-Poorly diff. adenoca.
-Insufficient anal function (DRE, manometry)
-Distant metastases
-Intestinal obstruction |
| Kuo, 2011 [26] |
-T1–3 |
-Infiltration EAS/LAR (even if submitted to nCRT with radiological clearance) |
| Martin, 2012 [33] (Review) |
-≤1 cm from anorectal ring |
-T4 tumors
-EAS/LAM infiltration
-Fixed tumors at DRE
-Poorly diff. adenoca.
-Fecal incontinence
-Distant metastases |
| Tokoro, 2013 [16] |
-T1–3
-Resectable metastases |
-T4 tumors
-Poorly diff. adenoca.
-Infiltrating gross appearance
-Fecal incontinence |
| Akagi, 2013 [17] |
-T1–3 (mobile tumors)
-≤4 cm from AV
-Well/moderately diff. adenoca.
-ECOG PS 0–2
-Good anal function |
-T4 tumor
-Fixed tumors
-Untreatable distant metastases
-Poorly diff. adenoca.
-Psychiatric disease
-Poor anal function (no discernable tone at DRE or the maximum squeeze pressure < 50 mmHg before operation)
-Liver cirrhosis, renal dysfunction, cardiac failure, and respiratory dysfunction |
| Akagi, 2013 [4] (Review) |
-T1–3 tumors
-30–35 mm from AV
-Independently to IAS invasion |
-As for Schiessel et al. |
| Saito, 2014 [28] |
-T1–4
-≤5 cm from AV |
-EAS/LAM infiltration
-Fecal incontinence |
Shirouzu, 2017 [34]
(Review) |
-T1–3
-1–5 cm AV
-Well-moderately diff. adenoca. |
-T4
-Fixed tumors
-EAS/LAM infiltration
-Untreatable distant metastases
-Poorly diff. adenoca.
-Poor anal function
-Severe preoperative pathologies (cardiac failure, liver cirrhosis, renal dysfunction, respiratory dysfunction)
-Psychiatric disease |
| Park, 2019 [20] |
-Tumor’s response to nCRT on restaging MRI
-Evaluation of ymrT stage and ymrCRM status |
-Poor nCRT responders |
| Piozzi, 2021 [21] |
-≤4 cm from AV
-After nCRT for cT3-T4
-(y)cT4 if curative resection is technically feasible at the pre-operative MRI
-Conversion from an ultra-low AR in case of involvement/threatening of the distal gross margin in the resected specimen or in case of stapler failure for any reason |
-EAS/LAM infiltration (at restaging MRI after nCRT)
-Abundant mucinous component
-Anal canal involvement below DL (requiring total ISR)
-Fecal incontinence
-Patient’s refusal |