Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Irene Gullo | + 1481 word(s) | 1481 | 2021-09-16 08:00:05 | | | |
| 2 | Rita Xu | -192 word(s) | 1289 | 2021-10-08 04:08:18 | | | | |
| 3 | Rita Xu | -192 word(s) | 1289 | 2021-10-08 04:08:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gullo, I. Adaptive Immune Landscape of Colorectal Adenoma-Carcinoma Sequence. Encyclopedia. Available online: https://encyclopedia.pub/entry/14853 (accessed on 07 February 2026).
Gullo I. Adaptive Immune Landscape of Colorectal Adenoma-Carcinoma Sequence. Encyclopedia. Available at: https://encyclopedia.pub/entry/14853. Accessed February 07, 2026.
Gullo, Irene. "Adaptive Immune Landscape of Colorectal Adenoma-Carcinoma Sequence" Encyclopedia, https://encyclopedia.pub/entry/14853 (accessed February 07, 2026).
Gullo, I. (2021, October 05). Adaptive Immune Landscape of Colorectal Adenoma-Carcinoma Sequence. In Encyclopedia. https://encyclopedia.pub/entry/14853
Gullo, Irene. "Adaptive Immune Landscape of Colorectal Adenoma-Carcinoma Sequence." Encyclopedia. Web. 05 October, 2021.
Copy Citation
The tumor immune microenvironment exerts a pivotal influence in tumor initiation and progression.
colorectal adenoma
colorectal adenocarcinoma
familial adenomatous polyposis
APC germline alterations
1. Introduction
The etiopathogenesis of most colorectal carcinomas (CRC) follows the conventional adenoma–carcinoma sequence (ACS), in which colorectal adenomas with low-grade dysplasia (LGD) and/or high-grade dysplasia (HGD) evolve to invasive adenocarcinoma (ADC), through a stepwise accumulation of genetic and epigenetic alterations. The earliest events in colorectal tumorigenesis involve alterations of the WNT signaling pathway, most frequently through somatic mutation of the adenomatous polyposis coli (APC) gene [1][2][3]. Germline mutations in the APC gene are the genetic cause of familial adenomatous polyposis (FAP), an autosomal dominant syndrome characterized by numerous adenomatous lesions in the colorectum, and early onset CRC.
The tumor immune microenvironment has a crucial role in the development and progression of colorectal precursor and invasive lesions [4]. Moreover, the amount, type, and location of tumor-infiltrating lymphocytes have been shown to have prognostic value in CRC patients [5][6][7]. With the widespread use of targeted immunotherapy in solid tumors, including CRC [8], there is a growing research interest in exploring the role of the tumor immune microenvironment also as a predictive biomarker. Currently, monoclonal antibodies against immune checkpoints, e.g., CTLA4, PD-1, and PD-L1, have been approved in microsatellite instability-high (MSI-H) CRCs and microsatellite-stable (MSS) tumors with high tumor mutation burden (TMB) [9][10], reflecting the immunogenicity of these tumor subtypes [11].
In the current body of literature, great attention has been given to the immune landscape of invasive ADC, whereas few studies have compared the tumor immune microenvironment of pre-invasive and malignant lesions, with contradictory findings [4][12][13][14][15][16]. Some studies have demonstrated a lower expression of Th1 cytokines, a shift to a Th2 cytokine profile [4][15], and a decrease in cytotoxic CD8+ T cell counts along ACS [17][18], whereas others have suggested an increase in the density of T lymphocytes, regulatory T cells, and macrophages during tumor progression [4][14][16]. Likewise, little information is available regarding the comparison of human colorectal neoplastic lesions arising from a sporadic or hereditary background, since most studies on hereditary cancer have used mouse models, especially APCmin mice [19]. Furthermore, most of the studies in human colorectal cancer focused on MSI tumors. A study by Liu, G. et al. showed that the number of CD3+, CD8+, CD4+ and FoxP3+ cells in the invasive margin or tumor stroma is higher in Lynch syndrome cases than in sporadic cases [20]. Therefore, an in-depth characterization of the human tumor immune landscape and its evolution along the colorectal ACS is needed, especially for MSS colorectal cancer.
2. Tumor Infiltration with Immune Cells Decreases throughout the Colorectal ACS
To investigate the evolution of the tumor immune microenvironment along the colorectal ACS, we analyzed the density and quality of the immune infiltration in adenomatous lesions with LGD, with HGD, and in invasive ADCs. By immunohistochemistry and counting representative areas, we analyzed and normalized the density of the overall population of CD3+ T lymphocytes per mm2, as well as the specific populations of CD4+ helper T cells, cytotoxic CD8+ T lymphocytes, Foxp3+ regulatory T cells, and CD57+ T lymphocytes/natural killer (NK) cells.
We observed an overall decrease in tumor-infiltrating immune cells along the colorectal ACS in both sporadic and hereditary FAP-related cohorts (Figure 1). Adenomatous lesions with LGD harbored higher counts of CD3+, CD4+, CD8+, and CD57+ immune cells when compared with adenomatous lesions with HGD and invasive ADCs, in both sporadic and hereditary FAP-related lesions. These findings were more pronounced in sporadic lesions, with p-values < 0.001 when adenomatous lesions with LGD were compared with ADCs. Although the density of Foxp3+ regulatory T cells followed the same decrease pattern in the sporadic context, this was not observed in lesions from FAP patients. As the spatial distribution of immune cell types within the tumors could be relevant in the interpretation of our results, we studied the immune cell density separately at the center and at the front of invasive ADCs. No differences were found between the two locations (data not shown) and the results on immune cell counts were considered as a whole.
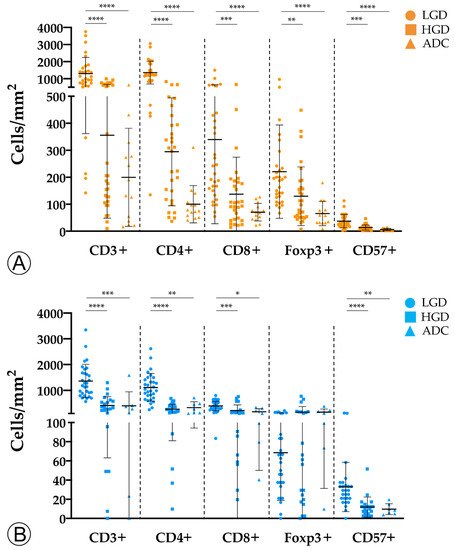
Figure 1. Counts of immune cells along the colorectal adenoma-carcinoma sequence in sporadic (A) and hereditary Familial Adenomatous Polyposis (FAP)-related (B) lesions. LGD, low-grade dysplasia; HGD, high-grade dysplasia; ADC, invasive adenocarcinoma; * = p < 0.05; ** = p < 0.01; *** = p < 0.001; **** = p < 0.0001.
To assess whether the tumor immune microenvironment could vary depending on the hereditary or sporadic background of the neoplastic lesions, we investigated the immune landscape between the two cohorts for LGD, HGD, and invasive ADC lesions (Figure 2).
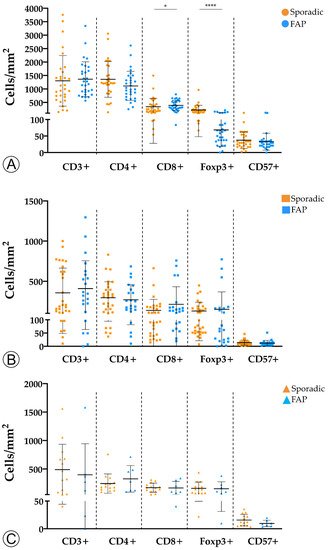
Figure 2. Comparison of immune cell counts between sporadic and hereditary Familial Adenomatous Polyposis (FAP)-related lesions in adenomatous lesions with low-grade dysplasia (A), adenomatous lesions with high-grade dysplasia (B), and invasive adenocarcinoma (C). * = p < 0.05; **** = p < 0.0001.
We found that adenomas with LGD from FAP patients displayed a marginal increase in CD8+ T cells (p < 0.05) and a significantly lower density of Foxp3+ (p < 0.0001) T cells when compared with sporadic adenomas with LGD. No differences were observed for the remaining adaptive immune cell populations, or subgroups of sporadic and hereditary lesions.
In conclusion, our findings suggest that colorectal neoplastic lesions trigger a host immune response, but become immunologically colder throughout the colorectal ACS. This was observed for both sporadic and hereditary lesions, although it was more pronounced in the former. In that regard, we would like to stress the higher density of Foxp3+ regulatory T cells in sporadic versus hereditary LGD lesions, suggesting that the host has a higher baseline immunological tolerance towards hereditary lesions.
3. TMB and MHC-I Expression Increase along the Colorectal ACS
We hypothesized that the decrease in immune cells counts along the ACS could be partially explained by the progressive loss of immunogenicity [21][22]. To explore this hypothesis, we evaluated TMB and the expression of the major histocompatibility complex class I (MHC-I) protein in tumor cells as surrogates to tumor antigenicity, and to the ability of tumor cells to present neoantigens to the immune system, respectively.
We found that TMB increased throughout the ACS in sporadic cases (Figure 3A), from the median value of 2.76 mutation/mega base pair (mut/Mbp) in LGD lesions to 15.76 mut/Mbp in ADC cases (p < 0.05). In contrast, this trend was not observed in hereditary FAP-related lesions (Figure 3B), where the average number of mut/Mbp remained constant along the ACS, despite the presence of some outlier samples. Likewise, we found that MHC-I expression in neoplastic cells progressively increased throughout the ACS in both FAP-related (p = 0.033) and sporadic (p = 0.025) lesions (Figure 4). MHC-I was expressed at the cell membrane, as well as in the cytoplasm of neoplastic cells (Figure 5).
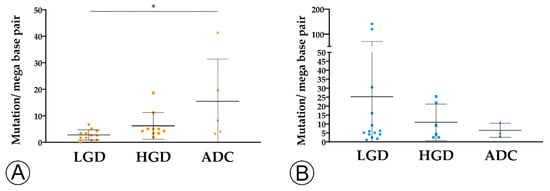
Figure 3. TMB analysis along the colorectal adenoma-carcinoma in sporadic (A) and hereditary Familial Adenomatous Polyposis (FAP)-related (B) lesions. * = p < 0.05. LGD, low-grade dysplasia (circles); HGD, high-grade dysplasia (squares); ADC, invasive adenocarcinoma (triangles); * = p < 0.05.
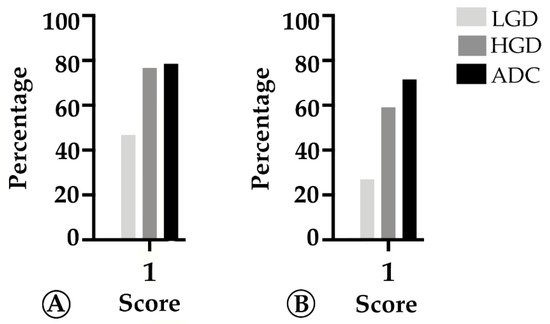
Figure 4. Percentage of cases in which MHC-I in tumor cells is >25% (score 1) along the colorectal adenoma-carcinoma sequence, in sporadic (A) and hereditary Familial Adenomatous Polyposis (FAP)-related (B) lesions. LGD, low-grade dysplasia; HGD, high-grade dysplasia; ADC, invasive adenocarcinoma.
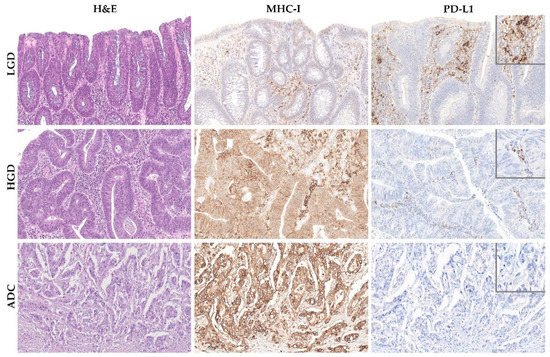
Figure 5. MHC-I and PD-L1 expression along the colorectal adenoma-carcinoma squence in representative examples of sporadic adenomatous lesion with low-grade dysplasia (LGD) (upper panel), sporadic adenomatous lesion with high-grade dysplasia (HGD) (middle panel), and sporadic invasive adenocarcinoma (ADC) (lower panel). Note the membranous and cytoplasmic immunoreactivity of MHC-I in neoplastic cells of HGD and invasive ADC lesions. Hematoxylin and eosin (left panel) and immunohistochemistry for MHC-I (middle panel) and PD-L1 (right panel), 40× amplification. The insets in the right panel represent higher magnification of PD-L1 immunohistochemistry, 400× amplification.
Overall, the increase in TMB in sporadic lesions, and the increase in MHC-I expression in both sporadic and FAP lesions along the ACS suggest that the observed decrease in immune cell counts cannot be explained by a loss of immunogenicity.
References
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767.
- Powell, S.M.; Zilz, N.; Beazer-Barclay, Y.; Bryan, T.M.; Hamilton, S.R.; Thibodeau, S.N.; Vogelstein, B.; Kinzler, K.W. APC mutations occur early during colorectal tumorigenesis. Nature 1992, 359, 235–237.
- Kinzler, K.W.; Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 1996, 87, 159–170.
- Cui, G.; Shi, Y.; Cui, J.; Tang, F.; Florholmen, J. Immune microenvironmental shift along human colorectal adenoma-carcinoma sequence: Is it relevant to tumor development, biomarkers and biotherapeutic targets? Scand. J. Gastroenterol. 2012, 47, 367–377.
- Nosho, K.; Baba, Y.; Tanaka, N.; Shima, K.; Hayashi, M.; Meyerhardt, J.A.; Giovannucci, E.; Dranoff, G.; Fuchs, C.S.; Ogino, S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: Cohort study and literature review. J. Pathol. 2010, 222, 350–366.
- Jakubowska, K.; Kisielewski, W.; Kanczuga-Koda, L.; Koda, M.; Famulski, W. Diagnostic value of inflammatory cell infiltrates, tumor stroma percentage and disease-free survival in patients with colorectal cancer. Oncol. Lett. 2017, 14, 3869–3877.
- Pages, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139.
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520.
- Golshani, G.; Zhang, Y. Advances in immunotherapy for colorectal cancer: A review. Ther. Adv. Gastroenterol. 2020, 13, 7527.
- Agarwal, P.; Le, D.T.; Boland, P.M. Immunotherapy in colorectal cancer. Adv. Cancer Res. 2021, 151, 137–196.
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421.
- Banner, B.F.; Savas, L.; Baker, S.; Woda, B.A. Characterization of the inflammatory cell populations in normal colon and colonic carcinomas. Virchows Arch. B Cell Pathol. 1993, 64, 213–220.
- McLean, M.H.; Murray, G.I.; Stewart, K.N.; Norrie, G.; Mayer, C.; Hold, G.L.; Thomson, J.; Fyfe, N.; Hope, M.; Mowat, N.A.; et al. The inflammatory microenvironment in colorectal neoplasia. PLoS ONE 2011, 6, e15366.
- Cui, G.; Yuan, A.; Vonen, B.; Florholmen, J. Progressive cellular response in the lamina propria of the colorectal adenoma-carcinoma sequence. Histopathology 2009, 54, 550–560.
- Cui, G.; Goll, R.; Olsen, T.; Steigen, S.E.; Husebekk, A.; Vonen, B.; Florholmen, J. Reduced expression of microenvironmental Th1 cytokines accompanies adenomas-carcinomas sequence of colorectum. Cancer Immunol. Immunother. 2007, 56, 985–995.
- Hua, W.; Yuan, A.; Zheng, W.; Li, C.; Cui, J.; Pang, Z.; Zhang, L.; Li, Z.; Goll, R.; Cui, G. Accumulation of FoxP3+ T regulatory cells in the tumor microenvironment of human colorectal adenomas. Pathol. Res. Pract. 2016, 212, 106–112.
- Cui, G. Immune battle at the premalignant stage of colorectal cancer: Focus on immune cell compositions, functions and cytokine products. Am. J. Cancer Res. 2020, 10, 1308–1320.
- Liu, F.; Hu, X.; Zimmerman, M.; Waller, J.L.; Wu, P.; Hayes-Jordan, A.; Lev, D.; Liu, K. TNFalpha cooperates with IFN-gamma to repress Bcl-xL expression to sensitize metastatic colon carcinoma cells to TRAIL-mediated apoptosis. PLoS ONE 2011, 6, e16241.
- Yang, J.; Wen, Z.; Li, W.; Sun, X.; Ma, J.; She, X.; Zhang, H.; Tu, C.; Wang, G.; Huang, D.; et al. Immune Microenvironment: New Insight for Familial Adenomatous Polyposis. Front. Oncol. 2021, 11, 570241.
- Liu, G.C.; Liu, R.Y.; Yan, J.P.; An, X.; Jiang, W.; Ling, Y.H.; Chen, J.W.; Bei, J.X.; Zuo, X.Y.; Cai, M.Y.; et al. The Heterogeneity Between Lynch-Associated and Sporadic MMR Deficiency in Colorectal Cancers. J. Natl. Cancer Inst. 2018, 110, 975–984.
- Morrison, B.J.; Steel, J.C.; Morris, J.C. Reduction of MHC-I expression limits T-lymphocyte-mediated killing of Cancer-initiating cells. BMC Cancer 2018, 18, 469.
- McGranahan, N.; Furness, A.J.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016, 351, 1463–1469.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Entry Collection:
Gastrointestinal Disease
Revisions:
3 times
(View History)
Update Date:
08 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




