Background: The tumor immune microenvironment exerts a pivotal influence in tumor initiation and progression. The aim of this study was to analyze the immune context of sporadic and familial adenomatous polyposis (FAP) lesions along the colorectal adenoma-carcinoma sequence (ACS).
Methods: We analyzed immune cell counts (CD3+, CD4+, CD8+, Foxp3+, and CD57+), tumor mutation burden (TMB), MHC-I expression and PD-L1 expression of 59 FAP and 74 sporadic colorectal lesions, encompassing adenomas with low-grade dysplasia (LGD) (30 FAP; 30 sporadic), adenomas with high-grade dysplasia (22 FAP; 30 sporadic), and invasive adenocarcinomas (7 FAP; 14 sporadic).
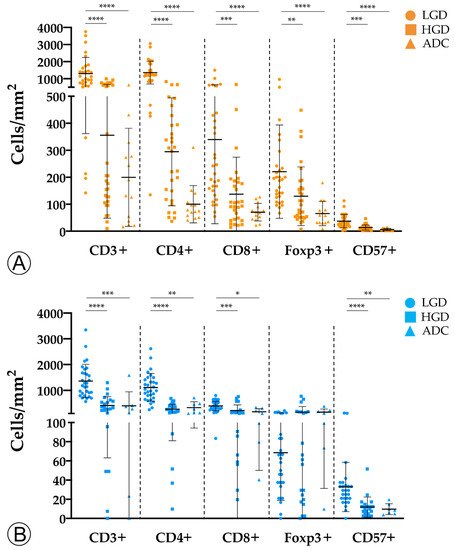
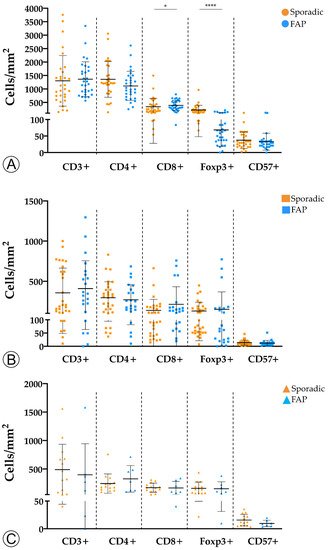
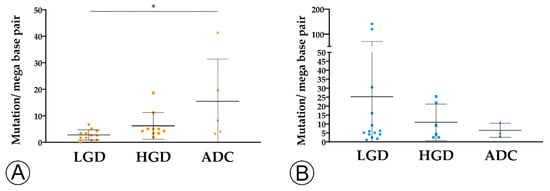
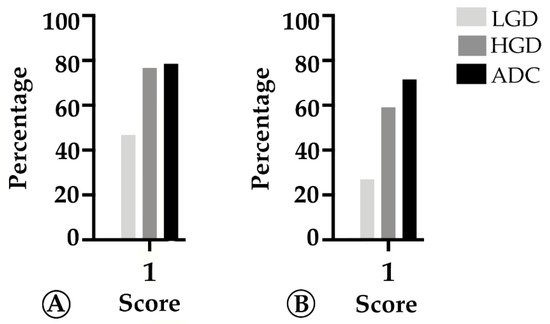
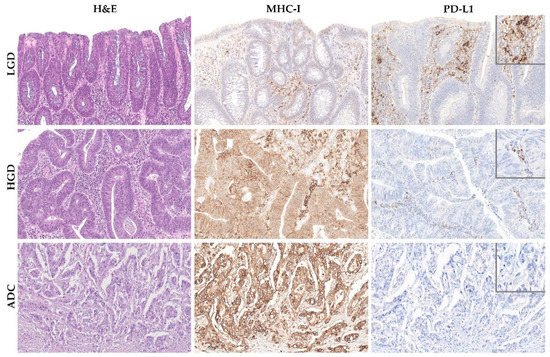
Results: The sporadic colorectal ACS was characterized by (1) a stepwise decrease in immune cell counts, (2) an increase in TMB and MHC-I expression, and (3) a lower PD-L1 expression. In FAP lesions, we observed the same patterns, except for an increase in TMB along the ACS. FAP LGD lesions harbored lower Foxp3+ T cell counts than sporadic LGD lesions. A decrease in PD-L1 expression occurred earlier in FAP lesions compared to sporadic ones.
Conclusions: The colorectal ACS is characterized by a progressive loss of adaptive immune infiltrate and by the establishment of a progressively immune cold microenvironment. These changes do not appear to be related with the loss of immunogenicity of tumor cells, or to the onset of an immunosuppressive tumor microenvironment.
- colorectal adenoma
- colorectal adenocarcinoma
- familial adenomatous polyposis
- APC germline alterations
1. Introduction
2. Tumor Infiltration with Immune Cells Decreases throughout the Colorectal ACS
3. TMB and MHC-I Expression Increase along the Colorectal ACS
This entry is adapted from the peer-reviewed paper 10.3390/ijms22189791
References
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767.
- Powell, S.M.; Zilz, N.; Beazer-Barclay, Y.; Bryan, T.M.; Hamilton, S.R.; Thibodeau, S.N.; Vogelstein, B.; Kinzler, K.W. APC mutations occur early during colorectal tumorigenesis. Nature 1992, 359, 235–237.
- Kinzler, K.W.; Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 1996, 87, 159–170.
- Cui, G.; Shi, Y.; Cui, J.; Tang, F.; Florholmen, J. Immune microenvironmental shift along human colorectal adenoma-carcinoma sequence: Is it relevant to tumor development, biomarkers and biotherapeutic targets? Scand. J. Gastroenterol. 2012, 47, 367–377.
- Nosho, K.; Baba, Y.; Tanaka, N.; Shima, K.; Hayashi, M.; Meyerhardt, J.A.; Giovannucci, E.; Dranoff, G.; Fuchs, C.S.; Ogino, S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: Cohort study and literature review. J. Pathol. 2010, 222, 350–366.
- Jakubowska, K.; Kisielewski, W.; Kanczuga-Koda, L.; Koda, M.; Famulski, W. Diagnostic value of inflammatory cell infiltrates, tumor stroma percentage and disease-free survival in patients with colorectal cancer. Oncol. Lett. 2017, 14, 3869–3877.
- Pages, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139.
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520.
- Golshani, G.; Zhang, Y. Advances in immunotherapy for colorectal cancer: A review. Ther. Adv. Gastroenterol. 2020, 13, 7527.
- Agarwal, P.; Le, D.T.; Boland, P.M. Immunotherapy in colorectal cancer. Adv. Cancer Res. 2021, 151, 137–196.
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421.
- Banner, B.F.; Savas, L.; Baker, S.; Woda, B.A. Characterization of the inflammatory cell populations in normal colon and colonic carcinomas. Virchows Arch. B Cell Pathol. 1993, 64, 213–220.
- McLean, M.H.; Murray, G.I.; Stewart, K.N.; Norrie, G.; Mayer, C.; Hold, G.L.; Thomson, J.; Fyfe, N.; Hope, M.; Mowat, N.A.; et al. The inflammatory microenvironment in colorectal neoplasia. PLoS ONE 2011, 6, e15366.
- Cui, G.; Yuan, A.; Vonen, B.; Florholmen, J. Progressive cellular response in the lamina propria of the colorectal adenoma-carcinoma sequence. Histopathology 2009, 54, 550–560.
- Cui, G.; Goll, R.; Olsen, T.; Steigen, S.E.; Husebekk, A.; Vonen, B.; Florholmen, J. Reduced expression of microenvironmental Th1 cytokines accompanies adenomas-carcinomas sequence of colorectum. Cancer Immunol. Immunother. 2007, 56, 985–995.
- Hua, W.; Yuan, A.; Zheng, W.; Li, C.; Cui, J.; Pang, Z.; Zhang, L.; Li, Z.; Goll, R.; Cui, G. Accumulation of FoxP3+ T regulatory cells in the tumor microenvironment of human colorectal adenomas. Pathol. Res. Pract. 2016, 212, 106–112.
- Cui, G. Immune battle at the premalignant stage of colorectal cancer: Focus on immune cell compositions, functions and cytokine products. Am. J. Cancer Res. 2020, 10, 1308–1320.
- Liu, F.; Hu, X.; Zimmerman, M.; Waller, J.L.; Wu, P.; Hayes-Jordan, A.; Lev, D.; Liu, K. TNFalpha cooperates with IFN-gamma to repress Bcl-xL expression to sensitize metastatic colon carcinoma cells to TRAIL-mediated apoptosis. PLoS ONE 2011, 6, e16241.
- Yang, J.; Wen, Z.; Li, W.; Sun, X.; Ma, J.; She, X.; Zhang, H.; Tu, C.; Wang, G.; Huang, D.; et al. Immune Microenvironment: New Insight for Familial Adenomatous Polyposis. Front. Oncol. 2021, 11, 570241.
- Liu, G.C.; Liu, R.Y.; Yan, J.P.; An, X.; Jiang, W.; Ling, Y.H.; Chen, J.W.; Bei, J.X.; Zuo, X.Y.; Cai, M.Y.; et al. The Heterogeneity Between Lynch-Associated and Sporadic MMR Deficiency in Colorectal Cancers. J. Natl. Cancer Inst. 2018, 110, 975–984.
- Morrison, B.J.; Steel, J.C.; Morris, J.C. Reduction of MHC-I expression limits T-lymphocyte-mediated killing of Cancer-initiating cells. BMC Cancer 2018, 18, 469.
- McGranahan, N.; Furness, A.J.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016, 351, 1463–1469.
