
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cornelia G. Palivan | + 1112 word(s) | 1112 | 2021-09-02 12:32:23 | | | |
| 2 | Dean Liu | Meta information modification | 1112 | 2021-09-08 04:36:54 | | |
Video Upload Options
Advances in peptide development have made peptide-assisted gene delivery more efficient in vitro and, in some instances, in small animal models. For example, cell and tissue selectivity could be greatly enhanced in the newest generation of CPPs. Other advances which allow for improved performance with regard to targeting and delivery of nucleic acids include adapting peptide sequences to facilitate escape or release from intracellular vesicles or respond to environmental stimuli for a controlled release of cargo, and the development of composite, multivalent peptide-based, or peptide-coupled structures.
1. Introduction
Introducing exogenous nucleic acids into human target cells has been receiving a great deal of attention for the treatment of several human diseases, in particular cancer and other genetic disorders. Despite a broad range of possible therapeutic approaches, the clinical success of gene therapy has yet to meet the expectations. The lack of efficacy and issues with clinical safety, in particular with viral vectors, which make up about 70% of vectors used in gene therapy, are the main reasons gene delivery systems fail in clinical trials.[1][2] This has led to the emergence of non-viral vector systems, such as liposomes and polymer supramolecular assemblies with better biological safety. However, their efficacy is predominantly hampered by insufficient localization of the therapeutic agents at the site of interest, both at the extracellular and intracellular level.[3] Owing to their remarkable potency, selectivity and low toxicity, peptides offer ideal alternatives to overcome these hurdles.[4]
The major shortcomings of most commonly used non-viral nucleic acid delivery systems, such as lipoplexes and polyplexes include nonspecific distribution, inefficient cytoplasmic delivery, and organelle targeting. In contrast, peptide-based nanocarriers, e.g., peptide nanoparticles, also called peptiplexes, or peptidic multicompartment micelles, and nano-assemblies equipped with peptides hold great promise as delivery platforms, since they can be tweaked to facilitate penetration of cell membranes and to localize to distinct subcellular compartments. In addition, peptides are easy to synthesize with a desired bioactivity, and, by multivalent presence, endow the nanocarrier with high avidity for the target.[5][6] Owing to the highly specific targeting capacity of corresponding peptides, therapeutic nanocarriers are able to pass through the cell membrane and reach the specific tissue and cells which results in enhanced intracellular distribution and extended therapeutic window.[7] Furthermore, smart delivery systems are promising options to provide solutions related to uncontrolled release of payloads: besides a biocompatible nanocarrier and suitable targeting moieties, these platforms include stimulus-responsive elements which endow them with triggered cargo release.[8]
2. Peptide-Guided Delivery of Nucleic Acids across Biological Barriers
Membrane active peptides interact with cellular membranes by traversing them, disrupting them or by residing at the membrane interface and fusing with them.[9] They are known to overcome site-specific delivery barriers and facilitate intracellular delivery of various bioactive cargos with low cytotoxicity.[9][10] Although there is a wide variety of membrane-active peptides, here we mainly discuss peptides for targeting nucleic acid delivery systems to specific cells and tissues, and peptides that assist in the delivery of nanocarriers across membrane barriers, such as cell penetrating peptides (CPPs), peptides facilitating endosomal escape and those that target nanocarriers to subcellular organelles.
In view of the fact that the nuclear membrane is the main barrier restricting transgene expression of most non-viral carriers, gene therapy is the obvious field for the application of nuclear targeting peptides.[11] The significance of incorporating NLS peptides into non-viral delivery systems that can adequately favor the genetic materials release into the nucleus manifests itself by expanding case studies.[12][13][14] Hereby, positively charged NLS peptides either are attached to the negatively charged DNA via electrostatic interactions or are covalently coupled to the phosphate backbone of the DNA or to the condensing agent of the non-viral vector.[11]
3. Peptide-Related Nano-Assemblies for Nucleic Acid Delivery
Peptides have great potential as self-assembly building blocks on account of primary and secondary structure variability. Depending on the design, they form various supramolecular assemblies, such as vesicles,[15] micelles,[16] nanotubes ,[17] nanofibers,[18] or nanoribbons.[19] Choosing corresponding peptide building blocks allows for tuning size and shape of the nano-assembly to obtain improved nanocarrier properties including cargo loading and delivery.[20][21] Moreover, the weak interactions involved in peptide self-assembly are sensitive to environmental conditions, enabling nano-assemblies to exhibit specific functionalities in response to different external stimuli, such as temperature, pH, redox state, enzymes, or even light.
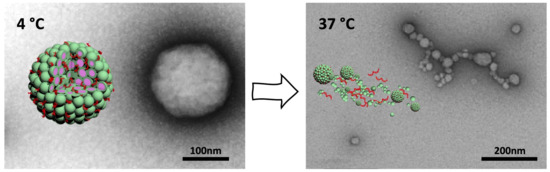
Light has received much attention as an external stimulus, as it provides spatiotemporal control that can be triggered remotely. By crosslinking peptides with specific light-absorbing molecules it is possible to obtain photo-responsive conjugates that allow for light-stimulated assembly of nanostructures or light-induced release of cargo molecules. Such light-sensitive conjugates of peptides and photosensitizers can serve as light-controllable phototherapeutic agents.[25]
4. Combinatorial Approach for Advanced Nucleic Acid Delivery
In view of gene therapy, endowing nanocarries with targeting features and stimuli-responsiveness that provides site-specific, triggerable control over cargo release could optimize delivery efficacy, and, at the same time, minimize adverse effects. Since the introduction of peptides as potential delivery system for a variety of therapeutic cargos, extensive research has focused on their application in gene therapy. To be suitably tailored for gene therapy, peptide-based nanocarriers must comply with issues of targeting, cellular uptake, and intracellular trafficking, all of which involve biological membranes and how they can be overcome. A combinatorial approach, e.g., designer peptides composed of cationic cell-penetrating and hydrophobic endosomal escape domains in combination with a gene carrier peptide composed of targeting and cationic DNA-binding domains affording triggered, site-specific (cytosol, nucleus, mitochondria) release of nucleic acids, may offer some improvement of efficacy. Other properties, including, but not limited to, low cytotoxicity, target specificity, biodegradability, and cost and time efficiency of synthesis greatly contribute to the potential of peptides in nanomedicine. Nevertheless, to broadly realize bench to bedside translation of peptide-related gene delivery systems, innovative technologies need to be pursued to achieve peptide-based nanocarriers that more specifically and efficiently deliver nucleic acids or nucleic acid modifying systems to the desired sites. In many cases, such nanocarriers would further benefit from either sustained or triggered delivery options.
References
- David B. Fogel; Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemporary Clinical Trials Communications 2018, 11, 156-164, 10.1016/j.conctc.2018.08.001.
- Attila A. Seyhan; Lost in translation: the valley of death across preclinical and clinical divide – identification of problems and overcoming obstacles. Translational Medicine Communications 2019, 4, 1-19, 10.1186/s41231-019-0050-7.
- Elvin Blanco; Haifa Shen; Mauro Ferrari; Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature Biotechnology 2015, 33, 941-951, 10.1038/nbt.3330.
- Markus Muttenthaler; Glenn F. King; David J. Adams; Paul F. Alewood; Trends in peptide drug discovery. Nature Reviews Drug Discovery 2021, 20, 309-325, 10.1038/s41573-020-00135-8.
- Alessandro Parodi; Claudia Corbo; Armando Cevenini; Roberto Molinaro; Roberto Palomba; Laura Pandolfi; Marco Agostini; Francesco Salvatore; Ennio Tasciotti; Enabling cytoplasmic delivery and organelle targeting by surface modification of nanocarriers. Nanomedicine 2015, 10, 1923-1940, 10.2217/nnm.15.39.
- Erkki Ruoslahti; Peptides as Targeting Elements and Tissue Penetration Devices for Nanoparticles. Advanced Materials 2012, 24, 3747-3756, 10.1002/adma.201200454.
- Woo-Jin Jeong; JiYoon Bu; Luke J. Kubiatowicz; Stephanie S. Chen; Youngsoo Kim; Seungpyo Hong; Peptide–nanoparticle conjugates: a next generation of diagnostic and therapeutic platforms?. Nano Convergence 2018, 5, 38, 10.1186/s40580-018-0170-1.
- S. Hossen; M. Khalid Hossain; M. Khairul Basher; M.N.H. Mia; M. T. Rahman; Jalal Uddin; Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. Journal of Advanced Research 2018, 15, 1-18, 10.1016/j.jare.2018.06.005.
- Fatma Gizem Avci; Berna Sariyar Akbulut; Elif Ozkirimli; Membrane Active Peptides and Their Biophysical Characterization. Biomolecules 2018, 8, 77, 10.3390/biom8030077.
- Shabnam Tarvirdipour; Cora-Ann Schoenenberger; Yaakov Benenson; Cornelia G. G. Palivan; A self-assembling amphiphilic peptide nanoparticle for the efficient entrapment of DNA cargoes up to 100 nucleotides in length. Soft Matter 2020, 16, 1678-1691, 10.1039/c9sm01990a.
- Marieke A. E. M. Van Der Aa; Enrico Mastrobattista; Ronald S. Oosting; Wim E. Hennink; Gerben A. Koning; Daan J. A. Crommelin; The Nuclear Pore Complex: The Gateway to Successful Nonviral Gene Delivery. Pharmaceutical Research 2006, 23, 447-459, 10.1007/s11095-005-9445-4.
- Christina Zelmer; Ludovit P. Zweifel; Larisa E. Kapinos; Ioana Craciun; Zekiye P. Güven; Cornelia G. Palivan; Roderick Y. H. Lim; Organelle-specific targeting of polymersomes into the cell nucleus. Proceedings of the National Academy of Sciences 2020, 117, 2770-2778, 10.1073/pnas.1916395117.
- Mikhail Durymanov; Joshua Reineke; Non-viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers. Frontiers in Pharmacology 2018, 9, 971, 10.3389/fphar.2018.00971.
- Regis Cartier; Regina Reszka; Utilization of synthetic peptides containing nuclear localization signals for nonviral gene transfer systems. Gene Therapy 2002, 9, 157-167, 10.1038/sj.gt.3301635.
- Dimitrios G. Fatouros; Dimitrios Lamprou; Andrew J. Urquhart; Spyros N. Yannopoulos; Ioannis Vizirianakis; Shuguang Zhang; Sotirios Koutsopoulos; Lipid-like Self-Assembling Peptide Nanovesicles for Drug Delivery. ACS Applied Materials & Interfaces 2014, 6, 8184-8189, 10.1021/am501673x.
- Ju Liang; Wen-Lan Wu; Xiao-Ding Xu; Ren-Xi Zhuo; Xian-Zheng Zhang; pH Responsive micelle self-assembled from a new amphiphilic peptide as anti-tumor drug carrier. Colloids and Surfaces B: Biointerfaces 2014, 114, 398-403, 10.1016/j.colsurfb.2013.10.037.
- Qinrong Wang; Xin Zhang; Jinhong Zheng; Daojun Liu; Self-assembled peptide nanotubes as potential nanocarriers for drug delivery. RSC Advances 2014, 4, 25461-25469, 10.1039/c4ra03304c.
- Chunqiu Zhang; Xiangdong Xue; Quan Luo; Yiwei Li; Keni Yang; Xiaoxi Zhuang; Yonggang Jiang; Jinchao Zhang; Junqiu Liu; Guozhang Zou; et al.Xing-Jie Liang Self-Assembled Peptide Nanofibers Designed as Biological Enzymes for Catalyzing Ester Hydrolysis. ACS Nano 2014, 8, 11715-11723, 10.1021/nn5051344.
- Meng Wang; Jiqian Wang; Peng Zhou; Jing Deng; Yurong Zhao; Yawei Sun; Wei Yang; Dong Wang; Zongyi Li; Xuzhi Hu; et al.Stephen M. KingSarah E. RogersHenry CoxThomas A. WaighJun YangJian Ren LuHai Xu Nanoribbons self-assembled from short peptides demonstrate the formation of polar zippers between β-sheets. Nature Communications 2018, 9, 5118, 10.1038/s41467-018-07583-2.
- Dirk De Bruyn Ouboter; Thomas B. Schuster; Alexandre Mantion; Wolfgang Meier; Hierarchical Organization of Purely Peptidic Amphiphiles into Peptide Beads. The Journal of Physical Chemistry C 2011, 115, 14583-14590, 10.1021/jp203048h.
- Dirk De Bruyn Ouboter; Thomas Schuster; Vijay Shanker; Markus Heim; Wolfgang Meier; Multicompartment micelle-structured peptide nanoparticles: A new biocompatible gene- and drug-delivery tool. Journal of Biomedical Materials Research Part A 2013, 102, 1155-1163, 10.1002/jbm.a.34778.
- Dennis W. P. M. Löwik; E. H. P. Leunissen; Maaike Van Den Heuvel; Morten B Hansen; Jan C. M. Van Hest; Stimulus responsive peptide based materials. Chemical Society Reviews 2010, 39, 3394-3412, 10.1039/b914342b.
- J. Andrew Mackay; Ashutosh Chilkoti; Temperature sensitive peptides: Engineering hyperthermia-directed therapeutics. International Journal of Hyperthermia 2008, 24, 483-495, 10.1080/02656730802149570.
- Severin J. Sigg; Viktoriia Postupalenko; Jason T. Duskey; Cornelia G. Palivan; Wolfgang Meier; Stimuli-Responsive Codelivery of Oligonucleotides and Drugs by Self-Assembled Peptide Nanoparticles. Biomacromolecules 2016, 17, 935-945, 10.1021/acs.biomac.5b01614.
- Manzar Abbas; Qianli Zou; Shukun Li; Xuehai Yan; Self-Assembled Peptide- and Protein-Based Nanomaterials for Antitumor Photodynamic and Photothermal Therapy. Advanced Materials 2017, 29, 1605021, 10.1002/adma.201605021.




