Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yong-Guang Gao | + 1930 word(s) | 1930 | 2022-01-14 04:01:25 | | | |
| 2 | Catherine Yang | Meta information modification | 1930 | 2022-01-21 09:10:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gao, Y. Non-Viral Delivery Systems. Encyclopedia. Available online: https://encyclopedia.pub/entry/18601 (accessed on 04 March 2026).
Gao Y. Non-Viral Delivery Systems. Encyclopedia. Available at: https://encyclopedia.pub/entry/18601. Accessed March 04, 2026.
Gao, Yong-Guang. "Non-Viral Delivery Systems" Encyclopedia, https://encyclopedia.pub/entry/18601 (accessed March 04, 2026).
Gao, Y. (2022, January 21). Non-Viral Delivery Systems. In Encyclopedia. https://encyclopedia.pub/entry/18601
Gao, Yong-Guang. "Non-Viral Delivery Systems." Encyclopedia. Web. 21 January, 2022.
Copy Citation
Non-viral delivery system simply refers to the use of non-viral vectors for drugs, genes or RNA delivery into cells. Non-viral vectors are safer, less toxic non-immunogenic alternatives to the viral vectors. They are easy to design, develop, and are inexpensive.
non-viral delivery system
1. Organic Delivery System
1.1. Cationic Lipids
Since the middle 1980s, synthetically-prepared, positively-charged cationic lipids were considered effective delivery vehicles for proteins and nucleic acids. Before this, they were used for purposes like membrane fusion mechanisms and protein studies [1][2]. Felgner and his coworkers in 1987 were the first to use cationic lipid (DOTMA) as a gene transfer vehicle [3]. Cationic lipids such as DOTMA (N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride), DOTAP(1,2-dioleoyl-3-trimethylammoniumpropane), DDAB (Didodecyldimethylammonium bromide), CTAB (Cetyltrimethylammonium bromide) (Figure 1) are considered to be the most appointed and well-studied non-viral vectors. Being positively charged they bond and self-assemble easily with negatively-charged nucleic acids making complexes called lipoplexes. They also bind to negatively charged cell membranes more readily than conventional liposomes [4].
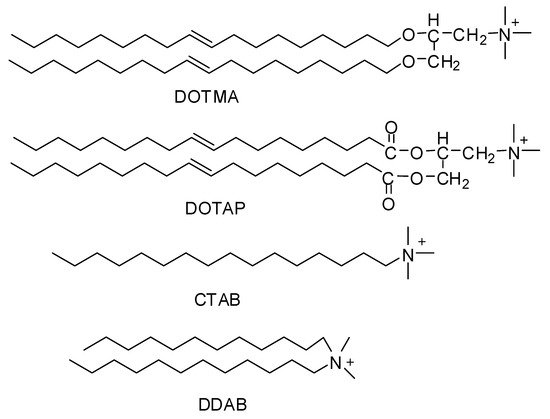
Figure 1. Chemical structures of cationic lipids.
Over the last ten years, a large number of cationic lipids have been developed and applied for the purpose of drug delivery especially nucleic acids. Some of them like lipofectamine and DOTAP are commercially available and frequently used as the gold standards in different cellular experiments for sufficient transfection efficiency [5]. They all have positively charged hydrophilic heads and hydrophobic tails, connected to each other with a linker. The hydrophilic head groups are mostly primary, secondary, tertiary amines, or quaternary ammonium salts, but some other groups like imidazole, guanidino, phosphorus, pyridinium, and arsenic groups have also been established. While the hydrophobic tails contain aliphatic chains, cholesterol, other types of steroid rings or rigid aromatic rings. Usually the linkage in between the head and tail is made by ester, ether carbamate, or amide groups [6]. This is the positively charged head that bind with negatively charged nucleic acids and linker that affect the rate of biodegradability.
Cationic lipids are comparatively less immunogenic, less toxic and easily producible. However, their transfection efficiency needs improvement. Moreover, they form aggregates in the blood causing toxicity and inflammatory response. But these drawbacks are treatable by designing ionizable cationic lipids with PEGlyated lipids in formulation. PEGlyation prevent the cationic lipids from opsonization by reticulo-endothelial system, and thus give the opportunity for prolonging in vivo circulation [7].
Cationic lipids have been used as successful delivery systems to different types of cells, including bone cells, endothelial cells, airway epithelial cells, muscle cells, placental cells, hepatocytes, tumors, and others. Recently, Attia and her colleagues developed a delivery system in the form of niosomes by using cationic lipid called 2,3-di(tetradecyloxy)propan-1-amine. They used these niosomes to deliver the BMP-7 gene to mesenchymal stem cells (MSCs). The BMP-7 gene actually codes for bone morphogenetic protein-7(BMP-7) that plays an important role in transforming mesenchymal stem cells (MSCs) into bone. An enhanced growth rate with extracellular matrix deposition and promoted alkaline phosphatase activity (ALP) was observed in transfected MSCs, thus suggesting the formation of osteoblasts-like cells. They concluded that their designed delivery tool can be used not only as efficient delivery system for BMP-7 but also as proliferating and bone forming cells for bone regeneration [8]. Xuelei Yin and his colleagues developed estrogen-functionalized liposomes grafted with glutathione-responsive sheddable chotooligosaccharides against osteosarcoma. They found that Chol-SS-COS/ES/DOX liposomes manifested higher cytotoxicity to MG63 osteosarcoma cells than to liver cells [9]. Moreover, our group recently designed a delivery system by deriving cationic lipids from [3]-aneN3 and modified it with fluorescent naphthalimide, oleic acid and octadecylamine. We found that all of them showed good transfection efficiency to osteoblastic cell line MC3T3-E1, MG63, HeLa, and HEK293 cells, but the one modified with naphthalimide showed even higher efficiency than lipofectamine 2000. Most importantly, it was successfully applied for in-situ monitoring of cellular uptake, DNA transportation, and release through non-invasive fluorescence imaging. Hence, we concluded that it can be used as a multifunctional non-viral delivery system for treating various bone disorders related to osteoblasts in future [10].
1.2. Cationic Polymers
Polymeric systems containing positive charges are known as cationic polymers. Being positively charged, they can bind with negatively-charged nucleic acids, proteins, and cell membranes through electrostatic interaction. When they are mixed with DNA they form complexes called polyplexes, usually more stable than lipoplexes [11]. They are considered to be excellent nucleic acids transfer vectors as they mediate the transfection through condensation of nucleic acids, facilitate their uptake by cells, protect them from nucleases, and help in endolysosomal escape. Moreover, they have been developed for use in other applications like drug delivery and tissue engineering.
In 1995, Boussif and his colleagues [12] were the first to use a cationic polymer called polyethylenimine (PEI) as a gene delivery vehicle. Now a great variety of cationic polymers have been synthesized and analyzed for their gene transfer ability. Cationic polymers may be either natural or synthetically developed. Natural cationic polymers include chitosan, cationic dextran, gelatin, cationic cellulose, and cationic cyclodextrin, while polyethylenimine (PEI), polyamidoamine (PAA), polyaminoester (PAE), poly-L-lysine (PLL), and poly dimethylaminoethylmethacrylate (PDMAEMA) (Figure 2) are the well-known synthetic cationic polymers [13]. Natural cationic polymers are generally non-toxic, less immunogenic, and biodegradable. Therefore, many of them like gelatin and cationic dextran have been approved by the FDA as safe biomaterials [14]. Synthetic polymers have the advantage of modification according to the situation by incorporating bioactive moieties and functional groups.
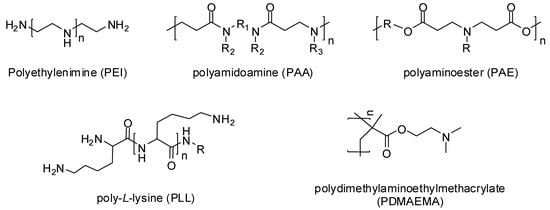
Figure 2. Chemical structures of cationic polymers.
Polyethylenimine (PEI) is the most important and highly used cationic polymer, synthesized in both linear and branched form with various molecular weights. It consists of reactive amino groups, and thus can be modified chemically for desirable physiochemical properties. The positively charged amino group of PEI can bind with negatively charged molecules including drugs and nucleic acids. However, they are somewhat cytotoxic and non-degradable, but they have the ability to form small and enzymatically stable polyplexes leading to high transfection efficiency [15].
Generally, cationic polymers with higher molecular weights and positive charges can act as efficient delivery systems but unfortunately they are limited due to their excessive cytotoxic effects [16]. Moreover, being highly positively charged, they can bind with anionic biomolecules in serum and thus may result in off-target side effects [17]. On the other hand, cationic polymers with low molecular weights possess nice cell tolerability but it is a pity that they are less efficient as compared to their higher molecular weight counterparts [18]. In order to break such a paradox between cytotoxicity and efficiency, recently, Liu and his fellows synthesized low molecular weight cationic polymers (LCPs) modified with coordinated zinc. They reported that Zn coordinated ligand bind with high affinity to the phosphate group in DNA that help the formulated polyplex in endosomal escape and higher transfection efficacy [19]. Recently, Bilecen and his colleagues developed a novel delivery system against osteoporosis by making a complex between PEI and RANK (Receptor activator of nuclear factor κ B) siRNA, and loaded it into poly(lactic acid-co-glycolic acid) (PLGA) nano-capsules. A reduction of about (47%) RANK mRNA levels was observed on osteoclast precursors which is directly proportional to the suppression of differentiation to mature osteoclasts. Hence they declared that this delivery system may reduce the number of mature osteoclasts that leads to the treatment of osteoporosis [20].
1.3. Cationic Peptides
Various cationic peptides are under investigation for use as safe and sound vehicles for targeted nucleic acids and negatively charged drug delivery. As they are rich in positively-charged lysine and arginine residues, therefore, they can bind with DNA efficiently and condense it into small compact particles. They are comparatively more advantageous because they have the ability to interact electrostatically with negatively charged nucleic acid or drugs, protect and target it to specific cell receptors, disrupt endosomal membrane, and deliver the cargo into nuclear localization [21]. Short peptides are readily attached to lipoplexes and polyplexes in order to give them directions for achieving specific targets in cell [6].
Recently, Mansure and his colleagues synthesized novel polymer–drug–peptide conjugates containing carboxymethylcellulose (CMC) conjugated by amide bonds with an anticancer drug called doxorubicin. In order to facilitate the targeting and internalization by cancer cells they dually functionalized the conjugate with L-arginine (R) and integrin-target receptor tripeptide (RGD). They tested and observed the bioconjugates against breast, bone, and brain cancer cell lines and reported them as ‘‘smart’’ delivery system to normal cells while highly toxic to cancer cells [22]. Sun and colleagues reacted cationic peptide SDSSD (Ser, Asp, Ser, Ser, Asp) with polyurethane (PU) nanomicells and developed a tremendous osteoblast-targeting delivery system, i.e., SDSSD-(PU) that specifically binds to osteoblasts through a surface protein called periostin. They utilized this system for the delivery of siRNA/microRNA to osteoblast and got successful results. They found out that SDSSD-PU could specifically target osteoblasts both in vitro and in vivo; therefore, it can be used as an effective osteoblast-targeting small nucleic acid delivery systems to treat skeletal disorders [23].
2. Inorganic Delivery System
2.1. Calcium Phosphates
In 1973, Graham and Van der Eb were the first to use calcium phosphates as non-viral vectors [24]. Now they are considered among the most prominent of non-viral delivery systems. They contain divalent cations, i.e., Ca2+ that form stable ionic complexes with negatively charged nucleic acids. These complexes are transferred to the cells through ion channel-mediated endocytosis. Although they possess low transfection efficiency, they are highly compatible and easily biodegradable. They are comparatively less toxic than commercial transfection reagents like Lipofectamine 2000, therefore they are considered as safe materials for transfection [25]. Moreover, they overwhelm the other targeting problems including endosomal escape, protection of the cargo in cytosol, and its delivery into the nucleus.
Calcium phosphate nanoparticles can be used in the treatment of various bone disorders by carrying drugs like bisphosphonates and other related drugs to the target sites [26]. Recently, Kentaro and his colleagues tested calcium phosphate cement (CPC) as a releasing and delivery material for a vancomycin antibiotic comparative to that of standard polymethylmethacrylate (PMMA) cement. They reported that calcium phosphate cement released more vancomycin both in vivo and in vitro for a longer time than PMMA, hence it can be used as an appropriate material for releasing antibiotics against postoperative infection [27].
2.2. Metal Nanoparticles
In the new era, nanotechnology has gained a lot of attention for developing new methods regarding diagnosis and treatment of diseases [28]. Various nanomaterials including grapheme, liposome, carbon nanotubes, magnetic nanoparticles, and metal nanoparticles have been utilized in many biomedical therapies. As they are nanosized, therefore it is easy to manipulate them at molecular level. Moreover, they possess large surface area, high compatibility, and strong antioxidant property [29].
Metal nanoparticles especially gold nanoparticles (Au NPs) are of prime importance for use in various biomedical applications as they can be designed in different sizes and different shapes. They are non-toxic and highly biocompatible. Moreover, they are electrostatically charged and hence can be functionalized by other biomolecules like nucleic acids and drugs [30]. They can be used to transfer multiple drugs molecules, nucleic acids or vaccines into the target sites with controlled release. They are able to make direct conjugates with different drugs molecules through ionic, covalent or physical absorption to treat endocellular diseases [31]. Lee and his colleagues made a conjugate of gold nanoparticle with alendronate of the bisphosphonate group and checked their inhibitory effects on the receptor activator of nuclear factor-κb ligand (RANKL)-induced osteoclastogenesis in bone marrow-derived macrophages. They observed that GNPs-ALD (gold nanoparticles conjugated with Alendronate) had suppressed osteoclast formation, thus declaring them as efficient agents for treating osteoporosis [32]. Apart from gold nanoparticles, iron oxide (Fe3O4) nanoparticles, which are safe, cheap, and chemically stable, can also be used to trigger osteoclast regulation. Actually, they possess high magnetic fields which are responsible for increasing local temperature, and in this way, suppress osteoclast functions to treat osteoporosis [33].
References
- Nikitenko, N.A.; Prassolov, V.S. Non-viral delivery and therapeutic application of small interfering rnas. Acta Nat. 2013, 5, 35–53.
- Engberts, J.B.F.N.; Hoekstra, D. Vesicle-forming synthetic amphiphiles. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1995, 1241, 323–340.
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417.
- Pezzoli, D.; Kajaste-Rudnitski, A.; Chiesa, R.; Candiani, G. Lipid-based nanoparticles as nonviral gene delivery vectors. Methods Mol. Biol. 2013, 1025, 269–279.
- Wang, W.; Balk, M.; Deng, Z.; Wischke, C.; Gossen, M.; Behl, M.; Ma, N.; Lendlein, A. Engineering biodegradable micelles of polyethylenimine-based amphiphilic block copolymers for efficient DNA and sirna delivery. J. Control. Release 2016, 242, 71–79.
- Al-Dosari, M.S.; Gao, X. Nonviral gene delivery: Principle, limitations, and recent progress. AAPS J. 2009, 11, 671–681.
- Musacchio, T.; Torchilin, V.P. Recent developments in lipid-based pharmaceutical nanocarriers. Front. Biosci. 2011, 16, 1388–1412.
- Attia, N.; Mashal, M.; Grijalvo, S.; Eritja, R.; Zarate, J.; Puras, G.; Pedraz, J.L. Stem cell-based gene delivery mediated by cationic niosomes for bone regeneration. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 521–531.
- Yin, X.; Feng, S.; Chi, Y.; Liu, J.; Sun, K.; Guo, C.; Wu, Z. Estrogen-functionalized liposomes grafted with glutathione-responsive sheddable chotooligosaccharides for the therapy of osteosarcoma. Drug Deliv. 2018, 25, 900–908.
- Gao, Y.G.; Alam, U.; Ding, A.X.; Tang, Q.; Tan, Z.L.; Shi, Y.D.; Lu, Z.L.; Qian, A.R. anen3-based lipid with naphthalimide moiety for enhanced gene transfection efficiency. Bioorg. Chem. 2018, 79, 334–340.
- Osada, K. Development of functional polyplex micelles for systemic gene therapy. Polym. J. 2014, 46, 469.
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natil. Acad. Sci. USA 1995, 92, 7297–7301.
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev. 2012, 41, 7147–7194.
- Zwiorek, K.; Bourquin, C.; Battiany, J.; Winter, G.; Endres, S.; Hartmann, G.; Coester, C. Delivery by cationic gelatin nanoparticles strongly increases the immunostimulatory effects of cpg oligonucleotides. Pharm. Res. 2008, 25, 551–562.
- Kichler, A.; Leborgne, C.; Coeytaux, E.; Danos, O. Polyethylenimine-mediated gene delivery: A mechanistic study. J. Gene Med. 2001, 3, 135–144.
- Huang, Q.; Li, S.; Ding, Y.F.; Yin, H.; Wang, L.H.; Wang, R. Macrocycle-wrapped polyethylenimine for gene delivery with reduced cytotoxicity. Biomater. Sci. 2018, 6, 1031–1039.
- Liu, S.; Yang, J.; Ren, H.; O’Keeffe-Ahern, J.; Zhou, D.; Zhou, H.; Chen, J.; Guo, T. Multifunctional oligomer incorporation: A potent strategy to enhance the transfection activity of poly(l-lysine). Biomater. Sci. 2016, 4, 522–532.
- Liu, S.; Zhou, D.; Yang, J.; Zhou, H.; Chen, J.; Guo, T. Bioreducible zinc(ii)-coordinative polyethylenimine with low molecular weight for robust gene delivery of primary and stem cells. J. Am. Chem. Soc. 2017, 139, 5102–5109.
- Liu, S.; Jia, H.; Yang, J.; Pan, J.; Liang, H.; Zeng, L.; Zhou, H.; Chen, J.; Guo, T. Zinc coordinated cationic polymers break up the paradox between low molecular weight and high transfection efficacy. Biomacromolecules 2018, 19, 4270–4276.
- Sezlev Bilecen, D.; Uludag, H.; Hasirci, V. Development of pei-rank sirna complex loaded plga nanocapsules for the treatment of osteoporosis. Tissue Eng. Part A 2019, 25, 34–43.
- Martin, M.E.; Rice, K.G. Peptide-guided gene delivery. AAPS J. 2007, 9, E18–E29.
- Mansur, A.A.P.; Carvalho, S.M.; Lobato, Z.I.P.; Leite, M.F.; Cunha, A.D.S., Jr.; Mansur, H.S. Design and development of polysaccharide-doxorubicin-peptide bioconjugates for dual synergistic effects of integrin-targeted and cell-penetrating peptides for cancer chemotherapy. Bioconjug. Chem. 2018, 29, 1973–2000.
- Sun, Y.; Ye, X.; Cai, M.; Liu, X.; Xiao, J.; Zhang, C.; Wang, Y.; Yang, L.; Liu, J.; Li, S.; et al. Osteoblast-targeting-peptide modified nanoparticle for sirna/microrna delivery. ACS Nano 2016, 10, 5759–5768.
- Graham, F.L.; van der Eb, A.J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 1973, 52, 456–467.
- Roy, I.; Mitra, S.; Maitra, A.; Mozumdar, S. Calcium phosphate nanoparticles as novel non-viral vectors for targeted gene delivery. Int. J. Pharm. 2003, 250, 25–33.
- Cao, X. Targeting osteoclast-osteoblast communication. Nat. Med. 2011, 17, 1344–1346.
- Uchida, K.; Sugo, K.; Nakajima, T.; Nakawaki, M.; Takano, S.; Nagura, N.; Takaso, M.; Urabe, K. In vivo release of vancomycin from calcium phosphate cement. BioMed Res. Int. 2018, 2018, 4560647.
- Akhter, S.; Ahmad, I.; Ahmad, M.Z.; Ramazani, F.; Singh, A.; Rahman, Z.; Ahmad, F.J.; Storm, G.; Kok, R.J. Nanomedicines as cancer therapeutics: Current status. Curr. Cancer Drug Targets 2013, 13, 362–378.
- Basarkar, A.; Singh, J. Nanoparticulate systems for polynucleotide delivery. Int. J. Nanomed. 2007, 2, 353–360.
- Verissimo, T.V.; Santos, N.T.; Silva, J.R.; Azevedo, R.B.; Gomes, A.J.; Lunardi, C.N. In vitro cytotoxicity and phototoxicity of surface-modified gold nanoparticles associated with neutral red as a potential drug delivery system in phototherapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 199–204.
- Chen, Y.H.; Tsai, C.Y.; Huang, P.Y.; Chang, M.Y.; Cheng, P.C.; Chou, C.H.; Chen, D.H.; Wang, C.R.; Shiau, A.L.; Wu, C.L. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol. Pharm. 2007, 4, 713–722.
- Lee, D.; Heo, D.N.; Kim, H.J.; Ko, W.K.; Lee, S.J.; Heo, M.; Bang, J.B.; Lee, J.B.; Hwang, D.S.; Do, S.H.; et al. Inhibition of osteoclast differentiation and bone resorption by bisphosphonate-conjugated gold nanoparticles. Sci. Rep. 2016, 6, 27336.
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in rankl-induced osteoclast differentiation. Blood 2005, 106, 852–859.
More
Information
Subjects:
Biochemical Research Methods
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
825
Revisions:
2 times
(View History)
Update Date:
21 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





