Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Magdalena Andrunik | + 1911 word(s) | 1911 | 2021-06-28 09:57:15 | | | |
| 2 | Vivi Li | + 135 word(s) | 2046 | 2021-07-07 03:50:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Andrunik, M. Removal of Pesticides from Waters. Encyclopedia. Available online: https://encyclopedia.pub/entry/11722 (accessed on 07 February 2026).
Andrunik M. Removal of Pesticides from Waters. Encyclopedia. Available at: https://encyclopedia.pub/entry/11722. Accessed February 07, 2026.
Andrunik, Magdalena. "Removal of Pesticides from Waters" Encyclopedia, https://encyclopedia.pub/entry/11722 (accessed February 07, 2026).
Andrunik, M. (2021, July 06). Removal of Pesticides from Waters. In Encyclopedia. https://encyclopedia.pub/entry/11722
Andrunik, Magdalena. "Removal of Pesticides from Waters." Encyclopedia. Web. 06 July, 2021.
Copy Citation
Pesticides are pollutants found in wastewater due to increasing agricultural activities over the years. Inappropriate dosing of pesticides results in the dispersal of active ingredients in the environment. The complete removal of pesticides from wastewater is an immediate concern due to their high toxicity and mobility. At present, adsorption is one of the most widely used methods for pesticide removal, in which synthetic zeolites and mesoporous silica materials are extensively applied. This article presents a systematic and comparative review of the applications and comparison of these adsorbents, based on the data reported in the literature.
agricultural wastes
water treatment
sorption
organic compounds
silicates
1. Introduction
Since the latter half of the nineteenth century, extensive agricultural use of plant protection products, which are referred to as “pesticides” (used henceforth in this paper), has been observed to pose a serious impact on soil, air, and water. As stated by the World Health Organization (WHO), the term “pesticide” is defined as any chemical compound that is used to kill pests (weeds, rodents, insects, fungi) [1]. The global demand for increased food production has not only led to significant deterioration of food quality, resulting in severe consequences on the environment but also caused public health issues due to overuse or misuse of pesticides [2][3]. It is assessed that more than 20% of the pesticides reach their nontarget species, as well as air, water, and soil [4]. Traces of these products are commonly detected in surface water, and more importantly, groundwater—a major source of drinking water on the world [5]. The presence of many types of pesticides and their derivatives in water is of great concern to the public and authorities, due to increased undesirable health effects resulted in the exposition on pesticides even at very low concentrations (pg/L to ng/L) [6].
Pesticides have greatly contributed to increasing agricultural yields by limiting pests and plant diseases and also by combating the insect-borne diseases in the human health sector [3][7]. For example, the production of food grains has increased dramatically in several countries since the implementation of pesticides. Although increases in productivity are attributed to different factors (e.g., use of fertilizers, better plants variations, and use of better machinery), pesticides have been an integral part of those processes by the reduction of losses from the weeds, diseases, and insect pests [8][9]. Furthermore, insecticides are the only way available to control the proliferation of deadly insect-borne diseases like malaria which results in an estimated 5000 deaths per day [10].
However, overuse and misuse of pesticides may have a negative impact on human health. Pesticides are used for controlling living species—they are biologically active substances that intervene with organisms and are characterized by different levels of toxicity [11]. These compounds are relatively stable and can bioaccumulate in living bodies. The toxicity of pesticides can be categorized as acute or chronic. Acute illness generally emerges after a short time of contact with the pesticide [12][13]. Suspected chronic effects resulting from regular exposure to small doses of certain pesticides may include birth defects, toxicity to fetus, genetic changes, blood, and nerve disorders. Furthermore, several studies have established a link between the exposure to pesticides and the frequency of chronic diseases that affect the nervous, reproductive, renal, cardiovascular, and respiratory systems in humans [14][15][16][17].
Based on their use and ability to kill organisms, pesticides can be classified as follows: insecticides, herbicides, rodenticides, fungicides, molluscicides, bactericides, avicides, virucides, algicides, acaricides, and miticides [9][18]. They can also be classified into four main groups according to the chemical nature of their active ingredients as follows: organochlorines, organophosphorus, carbamates, and pyrethrins and pyrethroids [19]. A brief description of those groups is presented in Table 1. In addition to the four main groups of pesticides mentioned above, there are few miscellaneous groups that are worth mentioning, such as phenoxyacetic acid (e.g., 2,4-D (2,4-dichlorophenoxyacetic acid) herbicide) or bipyridyls (e.g., paraquat and diquat herbicides). Inorganic pesticides are a minor category and include sulfur, copper, mercury, lead, and arsenic compounds. These pesticides are identified as extremely persistent and have caused serious problems of soil pollution in some areas; therefore, many of these are restricted [18][20][21].
Table 1. Characteristics of the main groups of pesticides.
Table 1. Characteristics of the main groups of pesticides.
| Pesticide | Mode of Action | Environmental Impact | Examples | References |
|---|---|---|---|---|
| Organochlorine | act as nervous system disruptors which leads to convulsions, paralysis, and death | long-term residual effect in the environment, resistant to most degradation processes | DDT (1,1,1-trichloro-2,2′bis(p-chlorophenyl)ethane), lindane, endosulfan | [9][18][22] |
| Organophosphorus | act as cholinesterase inhibitors causing a permanent overlay of acetylcholine neurotransmitters across a synapse which leads to paralysis and death | not persistent in the environment, susceptible to biodegradation | parathion, malathion, diazinon | [9][18][23] |
| Carbamates | act as cholinesterase inhibitors; mechanism of cholinesterase inhibition is species-specific and reversible | not persistent in the environment, susceptible to biodegradation | carbaryl, carbofuran, propoxur | [18][24] |
| Pyrethrins (natural) Pyrethroids (synthetic) | act by disrupting an insect’s nervous system which leads to a weakened state followed by death | not persistent in the environment, susceptible to biodegradation | permethrin, cypermethrin, deltamethrin | [18][25] |
Currently, the removal of pesticides and their derivatives from the environment is one of the worldwide environmental alarms. Due to the wide use of different types of pesticides, it is extremely difficult to develop a single universal method for their removal. Basically, three groups of methods are used for pesticide remediation: biological, chemical, and physical (Figure 1). Biological remediation results in the transformation of organic compounds into harmless products such as CO2 and H2O [26][27][28][29][30][31][32]. These methods are of low cost and believed to be more environmental-friendly compared to the physical and chemical remediation methods. In chemical remediation, pesticides are converted into harmless compounds through certain agents by the way of chemical reactions [33][34][35][36][37][38][39][40][41]. Chemical treatment is usually combined with physical remediation processes; however, the costs of these combined treatments are very high and vary depending on the matrix [42]. Physical remediation is based mostly on the process of adsorption, which is one of the most commonly used methods for water purification because of its capacity, efficiency, and applicability on a large scale [3][22][43][44][45][46][47][48][49].
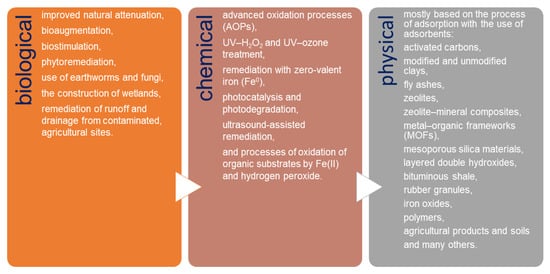
Figure 1. Methods used for pesticide remediation.
2. Characterization of Adsorbents
2.1. Mesoporous Silica Materials
Mesoporous silica materials are a new class of absorbents containing periodic arrays of channels and cavities [50]. The International Union of Pure and Applied Chemistry (IUPAC) defines them as materials with pore size in the range of 2–50 nm and exhibit an ordered arrangement of pores, which is responsible for their ordered structure [51][52]. The formation of those inorganic materials is based on the utilization of ordered surfactants as a template for the condensation of sodium silicate or silicon alkoxides around it [53]. Although the beginning of the synthesis of mesoscopic materials is dated back to the 1970s, mesoporous silica received attention only in the 1990s, when the Mobil Research and Development Corporation first synthesized mesoporous material from aluminosilicate gels with different morphological characteristics using the liquid-crystal template mechanism. Those materials were called Mobil Crystalline Materials or Mobil Composition of Matter (MCM) [51][52]. A few years later, silica nanoparticles with larger pores and thicker silica walls were produced at the University of California, Santa Barbara, which were named Santa Barbara Amorphous (SBA)-type materials [54]. Nowadays, there are various types of mesoporous silica materials available with different structural characteristics and functional groups [55].
The properties of mesoporous silica materials obtained depend strictly on the method used and the parameters of synthesis. These materials can be synthesized with different porosity, morphology, particle sizes, as well as with different functional groups, and hence, their physical and chemical properties can be tuned [56][57]. Usually, they have an enormous specific surface area (1000 m2/g or more), homogeneous pore distribution, and large volume and size of pores [58][59]. Generally, MCM-41 has a hexagonal structure with a pore diameter of 2.5–6 nm. MCM-48 has is cubic, whereas MCM-50 has a lamella-like arrangement [60]. SBA-type mesoporous silica materials differ from MCM type as they have larger pores with a size of 4.6–30 nm and thicker silica walls [61]. Based on the template used, SBA-based silica may be designated as SBA-11 (cubic), SBA-12 (three dimensional hexagonal), SBA-15 (hexagonal), and SBA-16 (cubic cage-structured) [51][62][63]. The structures of selected mesoporous silica materials are presented in the Figure S1.
Due to their diverse properties, mesoporous silica has a broad range of potential applications. In particular, they are used in medicine as, for example, delivery vehicles for pharmaceutical and biological molecules, host materials for bioimaging or biocatalytic agents, and platform materials for sensory or catalytic moieties [56][64][65][66]. They are also useful for the immobilization and separation of CO2, heavy metals, organic pollutants, volatile organic compounds, bioactive molecules, pigments, and dyes and thus play an important role in environmental protection [53][64][67][68][69]. Moreover, mesoporous silica materials attract a lot of academic interest because of their wide application in catalyst chemistry, electrochemistry, and energy storage [70][71][72][73].
2.2. Synthetic Zeolites
Zeolites include more than 50 aluminosilicate minerals with the general formula:
M2/nO∙Al2O3∙xSiO2∙yH2O (1)
where M is any alkali or alkaline earth atom, n is the charge on that atom, x is a number varying from 2 to 10, and y is a number varying from 2 to 7 [74]. Zeolites have a three-dimensional crystalline structure made of AlO4 and SiO4. The connection of the atoms forces the structure of the zeolite—four oxygen atoms are located at the corners of each tetrahedron and are shared with the adjoining crystal tetrahedral, and each tetrahedron in the framework contains silicon or alumina as its central atom [74][75][76][77]. Such orientation of atoms results in the development of the structure full of pores and empty voids formed as cages and channels [78]. The crystalline lattice structure of zeolites is characterized by unique lattice stability and allows ion exchange as well as the accommodation of water molecules, new cations, and small organic molecules. Molecules and particles occurring in the voids and pores are loosely bound, and therefore, all the mentioned processes are reversible with no damage caused to the zeolitic framework [74]. However, this feasibility is determined by the crystalline structures and chemical composition and of a particular zeolite. The type of zeolites formed depends on the temperature, pH pressure, concentration of the reagent solutions, process of activation and aging period, and the contents of SiO2 and Al2O3 in the raw materials [75].
The most important zeolites physical properties are their bulk density, specific surface area, specific gravity, radius and volume of pores, which can correlate with the porosity of the materials, and cation exchange capacity (CEC) [79][80][81]. The CEC and adsorption properties, pH, and loss on acid immersion of zeolites are some of the chemical properties characteristic of zeolites and depend on the chemical composition of the zeolite. In general, for zeolites, a raise in the Si/Al ratio (from 0.5 to infinity) leads to changes in various parameters—acid resistivity, thermal stability, and increase in hydrophobicity, while hydrophilicity, acid-site density, and cation concentration may decrease [80][82]. The properties of synthetic zeolites are also influenced by the method used for synthesis and its related parameters. Both the surface area and CEC of zeolites exhibit substantial variations with a raise in alkali concentration and reaction time [79]. For example, the surface area tends to raise with an increase in the concentration of alkaline solution and the reaction time, while the CEC decreases with increasing concentration of alkaline solution; however, CEC also fluctuates arbitrarily with a raise in reaction time, which is usually ascribed to the changes in the size and volume of the pores [74]. The frameworks of selected zeolites are presented in the Figure S2.
The potential applications of zeolitic materials in industrial field depends on their type and properties. Zeolites (especially those with high CEC) can be applied in water purification. In particular, the use of zeolites has been extensively tested in solutions containing heavy metals and ammonium [83][84][85], which are commonly used as molecular sieves in gas purification technology [84][86]. Zeolites, as the most important solid catalysts, are used in traditional petrochemical industries, especially in cracking, isomerization, and hydrocarbon synthesis [87]. These materials are also used as detergent builders, as in contrast to conventional detergent builders, they are more environmentally friendly due to their ability to lower the hardness of water and insolubility [88]. Moreover, Canpolat et al. [89] showed that the use of zeolite as a replacement material in the production of cement increased the compressive strength of the obtained cement products. Besides these applications, zeolites play a significant role in many sustainable processes, especially in the fields associated with renewable energy and environmental protection, such as biomass conversion, fuel cell production, thermal energy storage, agriculture, or biomedicine [87][90][91].
References
- Ioannides, C. Public-health impact of pesticides used in agriculture—WHO. J. R. Soc. Health 1991, 111, 206.
- Abhilash, P.C.; Singh, N. Pesticide use and application: An Indian scenario. J. Hazard. Mater. 2009, 165, 1–12.
- Ahmad, T.; Rafatullah, M.; Ghazali, A.; Sulaiman, O.; Hashim, R.; Ahmad, A. Removal of Pesticides from Water and Wastewater by Different Adsorbents: A Review. J. Environ. Sci. Health Part C-Environ. Carcinog. Ecotoxicol. Rev. 2010, 28, 231–271.
- Miller, G.T. Biodiversity. In Sustaining the Earth; Thompson Learning, Inc.: Pacific Grove, CA, USA, 1994; pp. 211–216. ISBN 9780495556879.
- Loos, R.; Gawlik, B.M.; Locoro, G.; Rimaviciute, E.; Contini, S.; Bidoglio, G. EU-wide survey of polar organic persistent pollutants in European river waters. Environ. Pollut. 2009, 157, 561–568.
- Skinner, J.A.; Lewis, K.A.; Bardon, K.S.; Tucker, P.; Catt, J.A.; Chambers, B.J. An overview of the environmental impact of agriculture in the UK. J. Environ. Manag. 1997, 50, 111–128.
- Cooper, J.; Dobson, H. The benefits of pesticides to mankind and the environment. Crop Prot. 2007, 26, 1337–1348.
- Bowles, R.G.; Webster, J.P.G. Some problems associated with the analysis of the costs and benefits of pesticides. Crop Prot. 1995, 14, 593–600.
- Tadeo, J.L. Analysis of Pesticides in Food and Environmental Samples, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781138486034.
- Rogan, W.J.; Chen, A.M. Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT). Lancet 2005, 366, 763–773.
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535.
- Lekei, E.; Ngowi, A.V.; Kapeleka, J.; London, L. Acute pesticide poisoning amongst adolescent girls and women in northern Tanzania. BMC Public Health 2020, 20, 8.
- Yu, S.Y.; Gao, Y.X.; Walline, J.; Lu, X.; Zhao, L.N.; Huang, Y.X.; Tao, J.; Yu, A.Y.; Ta, N.; Xiao, R.J.; et al. Role of penehyclidine in acute organophosphorus pesticide poisoning. World J. Emerg. Med. 2020, 11, 37–47.
- Mostafalou, S.; Abdollahi, M. Current concerns on genotoxicity of pesticides. Int. J. Pharmacol. 2012, 8, 473–474.
- Mostafalou, S.; Abdollahi, M. The Role of Environmental Pollution of Pesticides in Human Diabetes. Int. J. Pharmacol. 2012, 8, 139–140.
- Bhardwaj, J.K.; Mittal, M.; Saraf, P.; Kumari, P. Pesticides induced oxidative stress and female infertility: A review. Toxin Rev. 2020, 39, 1–13.
- Ionel, I.L.; Mara, G.; Stefania, R.; Margarita, G.O.; Corina, P. A hazard to human health—Pesticide residues in some vegetal and animal foodstuff. J. Biotechnol. 2019, 305, S22–S23.
- Zacharia, J.T. Identity, Physical and Chemical Properties of Pesticides. In Pesticides in the Modern World: Trends in Pesticides Analysis; Stoytcheva, M., Ed.; IntechOpen: Rijeka, Croatia, 2011; ISBN 978-953-307-437-5.
- Buchel, K.H. Chemistry of pesticides. J. Environ. Sci. Heal.—Part B Pestic. Food Contam. Agric. Wastes 1983, B18, 419–643.
- Khairy, M.; Ayoub, H.A.; Rashwan, F.A.; Abdel-Hafez, H.F. Chemical modification of commercial kaolin for mitigation of organic pollutants in environment via adsorption and generation of inorganic pesticides. Appl. Clay Sci. 2018, 153, 124–133.
- Strekopytov, S.; Brownscombe, W.; Lapinee, C.; Sykes, D.; Spratt, J.; Jeffries, T.E.; Jones, C.G. Arsenic and mercury in bird feathers: Identification and quantification of inorganic pesticide residues in natural history collections using multiple analytical and imaging techniques. Microchem. J. 2017, 130, 301–309.
- Zolgharnein, J.; Shahmoradi, A.; Ghasemi, J. Pesticides Removal Using Conventional and Low-Cost Adsorbents: A Review. Clean-Soil Air Water 2011, 39, 1105–1119.
- MacBean, C. Pesticides Manual, 1st ed.; British Crop Protection Council: London, UK, 2012.
- Drum, C. Soil Chemistry of Pesticides; PPG Industries, Inc.: Pittsburgh, PA, USA, 1980.
- Soderlund, D.M. Toxicology and Mode of Action of Pyrethroid Insecticides. In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Krieger, R., Ed.; Elsevier Academic Press Inc.: Geneva, NY, USA, 2010; pp. 1665–1686. ISBN 978-0-08-092201-0.
- Nwankwegu, A.S.; Onwosi, C.O. Bioremediation of gasoline contaminated agricultural soil by bioaugmentation. Environ. Technol. Innov. 2017, 7, 1–11.
- Marican, A.; Durán-Lara, E.F. A review on pesticide removal through different processes. Environ. Sci. Pollut. Res. 2018, 25, 2051–2064.
- Helbling, D.E. Bioremediation of pesticide-contaminated water resources: The challenge of low concentrations. Curr. Opin. Biotechnol. 2015, 33, 142–148.
- Vymazal, J.; Bfezinova, T. The use of constructed wetlands for removal of pesticides from agricultural runoff and drainage: A review. Environ. Int. 2015, 75, 11–20.
- Rodriguez-Campos, J.; Dendooven, L.; Alvarez-Bernal, D.; Contreras-Ramos, S.M. Potential of earthworms to accelerate removal of organic contaminants from soil: A review. Appl. Soil Ecol. 2014, 79, 10–25.
- Moore, M.T.; Tyler, H.L.; Locke, M.A. Aqueous pesticide mitigation efficiency of Typha latifolia (L.), Leersia oryzoides (L.) Sw., and Sparganium americanum Nutt. Chemosphere 2013, 92, 1307–1313.
- Maqbool, Z.; Hussain, S.; Imran, M.; Mahmood, F.; Shahzad, T.; Ahmed, Z.; Azeem, F.; Muzammil, S. Perspectives of using fungi as bioresource for bioremediation of pesticides in the environment: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 16904–16925.
- Hamby, D.M. Site remediation techniques supporting environmental restoration activities—A review. Sci. Total Environ. 1996, 191, 203–224.
- Cheng, M.; Zeng, G.M.; Huang, D.L.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598.
- Khan, F.I.; Husain, T.; Hejazi, R. An overview and analysis of site remediation technologies. J. Environ. Manag. 2004, 71, 95–122.
- Rani, S.; Sud, D. Role of enhanced solar radiation for degradation of triazophos pesticide in soil matrix. Sol. Energy 2015, 120, 494–504.
- Shea, P.J.; Machacek, T.A.; Comfort, S.D. Accelerated remediation of pesticide-contaminated soil with zerovalent iron. Environ. Pollut. 2004, 132, 183–188.
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218.
- Matouq, M.A.; Al-Anber, Z.A.; Tagawa, T.; Aljbour, S.; Al-Shannag, M. Degradation of dissolved diazinon pesticide in water using the high frequency of ultrasound wave. Ultrason. Sonochem. 2008, 15, 869–874.
- Fukuzumi, S.; Lee, Y.-M.; Jung, J.; Nam, W. Thermal and photocatalytic oxidation of organic substrates by dioxygen with water as an electron source. Green Chem. 2018, 20, 948–963.
- Barbusinski, K. Fenton reaction—controversy concerning the chemistry. Ecol. Chem. Eng. S-Chemia I Inz. Ekol. S 2009, 16, 347–358.
- Brooks, K.M.; Stierns, A.R.; Mahnken, C.V.W.; Blackburn, D.B. Chemical and biological remediation of the benthos near Atlantic salmon farms. Aquaculture 2003, 219, 355–377.
- Bonvin, F.; Jost, L.; Randin, L.; Bonvin, E.; Kohn, T. Super-fine powdered activated carbon (SPAC) for efficient removal of micropollutants from wastewater treatment plant effluent. Water Res. 2016, 90, 90–99.
- Rodriguez-Cruz, M.S.; Sanchez-Martin, M.J.; Andrades, M.S.; Sanchez-Camazano, M. Modification of clay barriers with a cationic surfactant to improve the retention of pesticides in soils. J. Hazard. Mater. 2007, 139, 363–372.
- Legrouri, A.; Lakraimi, M.; Barroug, A.; De Roy, A.; Besse, J.P. Removal of the herbicide 2,4-dichlorophenoxyacetate from water to zinc-aluminium-chloride layered double hydroxides. Water Res. 2005, 39, 3441–3448.
- Akcay, G.; Akcay, M.; Yurdakoc, K. The characterization of prepared organomontmorillonite (DEDMAM) and sorption of phenoxyalkanoic acid herbicides from aqueous solution. J. Colloid Interface Sci. 2006, 296, 428–433.
- Wang, S.B.; Wu, H.W. Environmental-benign utilisation of fly ash as low-cost adsorbents. J. Hazard. Mater. 2006, 136, 482–501.
- Singh, N. Adsorption of herbicides on coal fly ash from aqueous solutions. J. Hazard. Mater. 2009, 168, 233–237.
- Estevinho, B.N.; Martins, I.; Ratola, N.; Alves, A.; Santos, L. Removal of 2,4-dichlorophenol and pentachlorophenol from waters by sorption using coal fly ash from a Portuguese thermal power plant. J. Hazard. Mater. 2007, 143, 535–540.
- Ozin, G.A.; Kresge, C.T.; Yang, H. Nucleation, growth and form of mesoporous silica: Role of defects and a language of shape. Stud. Surf. Sci. Catal. 1998, 117, 119–127.
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 49.
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.T.; Lin, V.S.Y. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853.
- Cecilia, J.A.; Tost, R.M.; Millan, M.R. Mesoporous Materials: From Synthesis to Applications. Int. J. Mol. Sci. 2019, 20, 3213.
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552.
- Katiyar, A.; Yadav, S.; Smirniotis, P.G.; Pinto, N.G. Synthesis of ordered large pore SBA-15 spherical particles for adsorption of biomolecules. J. Chromatogr. A 2006, 1122, 13–20.
- Asefa, T.; Tao, Z.M. Mesoporous silica and organosilica materials—Review of their synthesis and organic functionalization. Can. J. Chem. 2012, 90, 1015–1031.
- Chen, L.; Zhou, X.J.; He, C.L. Mesoporous silica nanoparticles for tissue-engineering applications. Wiley Interdiscip. Rev. Nanobiotechnology 2019, 11, 22.
- Laskowski, L.; Laskowska, M.; Vila, N.; Schabikowski, M.; Walcarius, A. Mesoporous Silica-Based Materials for Electronics-Oriented Applications. Molecules 2019, 24, 31.
- Jehng, J.M.; Tung, W.C.; Kuo, C.H. The formation mechanisms of multi-wall carbon nanotubes over the Ni modified MCM-41 catalysts. J. Porous Mater. 2008, 15, 43–51.
- Oye, G.; Sjoblom, J.; Stocker, M. Synthesis, characterization and potential applications of new materials in the mesoporous range. Adv. Colloid Interface Sci. 2001, 89, 439–466.
- Zhao, D.Y.; Huo, Q.S.; Feng, J.L.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 10546.
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251.
- Nishihara, H.; Kyotani, T. Templated nanocarbons for energy storage. Adv. Mater. 2012, 24, 4473–4498.
- Da’na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Microporous Mesoporous Mater. 2017, 247, 145–157.
- Gargiulo, N.; Cusano, A.M.; Causa, F.; Caputo, D.; Netti, P.A. Silver-containing mesoporous bioactive glass with improved antibacterial properties. J. Mater. Sci. Med. 2013, 24, 2129–2135.
- Pourshahrestani, S.; Zeimaran, E.; Kadri, N.A.; Gargiulo, N.; Samuel, S.; Naveen, S.V.; Kamarul, T.; Towler, M.R. Gallium-containing mesoporous bioactive glass with potent hemostatic activity and antibacterial efficacy. J. Mater. Chem. B 2016, 4, 71–86.
- Gargiulo, N.; Verlotta, A.; Peluso, A.; Aprea, P.; Caputo, D. Modeling the performances of a CO2 adsorbent based on polyethylenimine-functionalized macro-/mesoporous silica monoliths. Microporous Mesoporous Mater. 2015, 215, 1–7.
- Serna-Guerrero, R.; Sayari, A. Applications of pore-expanded mesoporous silica. 7. Adsorption of volatile organic compounds. Environ. Sci. Technol. 2007, 41, 4761–4766.
- Walcarius, A.; Mercier, L. Mesoporous organosilica adsorbents: Nanoengineered materials for removal of organic and inorganic pollutants. J. Mater. Chem. 2010, 20, 4478–4511.
- Li, C.C.; Qiao, X.C. A new approach to prepare mesoporous silica using coal fly ash. Chem. Eng. J. 2016, 302, 388–394.
- Balcar, H.; Cejka, J. Mesoporous molecular sieves as advanced supports for olefin metathesis catalysts. Coord. Chem. Rev. 2013, 257, 3107–3124.
- Hasanzadeh, M.; Shadjou, N.; Eskandani, M.; de la Guardia, M.; Omidinia, E. Mesoporous silica materials for use in electrochemical immunosensing. TrAC-Trends Anal. Chem. 2013, 45, 93–106.
- Motahar, S.; Nikkam, N.; Alemrajabi, A.A.; Khodabandeh, R.; Toprak, M.S.; Muhammed, M. A novel phase change material containing mesoporous silica nanoparticles for thermal storage: A study on thermal conductivity and viscosity. Int. Commun. Heat Mass Transf. 2014, 56, 114–120.
- Jha, B.; Singh, D.N. A review on synthesis, characterization and industrial applications of flyash zeolites. J. Mater. Educ. 2011, 33, 65–132.
- Scott, J.; Guang, D.; Naeramitmarnsuk, K.; Thabuot, M.; Amal, R. Zeolite synthesis from coal fly ash for the removal of lead ions from aqueous solution. J. Chem. Technol. Biotechnol. 2002, 77, 63–69.
- Rayalu, S.; Meshram, S.U.; Hasan, M.Z. Highly crystalline faujasitic zeolites from flyash. J. Hazard. Mater. 2000, 77, 123–131.
- Baerlocher, C.; Meier, W.M.; Olson, D.H. Atlas of Zeolite Framework Types, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2001; ISBN 978-0-444-50701-3.
- Qiu, L.Y.; Murashov, V.; White, M.A. Zeolite 4A: Heat capacity and thermodynamic properties. Solid State Sci. 2000, 2, 841–846.
- Singh, D.N.; Kolay, P.K. Simulation of ash-water interaction and its influence on ash characteristics. Prog. Energy Combust. Sci. 2002, 28, 267–299.
- Beving, D.E.; O’Neill, C.R.; Yan, Y.S. Hydrophilic and antimicrobial low-silica-zeolite LTA and high-silica-zeolite MFI hybrid coatings on aluminum alloys. Microporous Mesoporous Mater. 2008, 108, 77–85.
- Kumar, P.; Mal, N.; Oumi, Y.; Yamana, K.; Sano, T. Mesoporous materials prepared using coal fly ash as the silicon and aluminium source. J. Mater. Chem. 2001, 11, 3285–3290.
- Davis, M.E.; Lobo, R.F. Zeolite and Molecular Sieve Synthesis. Chem. Mater. 1992, 4, 756–768.
- Querol, X.; Moreno, N.; Umana, J.C.; Alastuey, A.; Hernandez, E.; Lopez-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423.
- Querol, X.; Moreno, N.; Umana, J.C.; Juan, R.; Hernandez, S.; Fernandez-Pereira, C.; Ayora, C.; Janssen, M.; Garcia-Martinez, J.; Linares-Solano, A.; et al. Application of zeolitic material synthesised from fly ash to the decontamination of waste water and flue gas. J. Chem. Technol. Biotechnol. 2002, 77, 292–298.
- Querol, X.; Alastuey, A.; Moreno, N.; Alvarez-Ayuso, E.; Garcia-Sanchez, A.; Cama, J.; Ayora, C.; Simon, M. Immobilization of heavy metals in polluted soils by the addition of zeolitic material synthesized from coal fly ash. Chemosphere 2006, 62, 171–180.
- Srinivasan, A.; Grutzeck, M.W. The adsorption of SO2 by zeolites synthesized from fly ash. Environ. Sci. Technol. 1999, 33, 1464–1469.
- Li, Y.; Li, L.; Yu, J. Applications of Zeolites in Sustainable Chemistry. Chem 2017, 3, 928–949.
- Udhoji, J.S.; Bansiwal, A.K.; Meshram, S.U.; Rayalu, S.S. Improvement in optical brightness of fly ash based zeolite-A for use as detergent builder. J. Sci. Ind. Res. 2005, 64, 367–371.
- Canpolat, F.; Yilmaz, K.; Kose, M.M.; Sumer, M.; Yurdusev, M.A. Use of zeolite, coal bottom ash and fly ash as replacement materials in cement production. Cem. Concr. Res. 2004, 34, 731–735.
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and actual uses of zeolites in crop protection. Pest Manag. Sci. 2015, 71, 1355–1367.
- Anfray, C.; Dong, B.A.; Komaty, S.; Mintova, S.; Valable, S. Acute Toxicity of Silver Free and Encapsulated in Nanosized Zeolite for Eukaryotic Cells. ACS Appl. Mater. Interfaces 2017, 9, 13849–13854.
More
Information
Subjects:
Chemistry, Organic; Environmental Sciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
07 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




