
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Graca Minas | + 1868 word(s) | 1868 | 2021-05-19 06:20:49 | | | |
| 2 | Lindsay Dong | Meta information modification | 1868 | 2021-05-21 11:44:20 | | | | |
| 3 | Lindsay Dong | Meta information modification | 1868 | 2021-05-21 11:45:51 | | |
Video Upload Options
Three-dimensional (3D) in vitro models, such as organ-on-a-chip platforms, are an emerging and effective technology that allows the replication of the function of tissues and organs, bridging the gap amid the conventional models based on planar cell cultures or animals and the complex human system. Hence, they have been increasingly used for biomedical research, such as drug discovery and personalized healthcare. A promising strategy for their fabrication is 3D printing, a layer-by-layer fabrication process that allows the construction of complex 3D structures.
1. Introduction
Over the last decades, conventional manufacturing techniques, such as photo-patterning, self-assembly, and soft lithography, have been used for the manufacturing of microfluidic devices and also OoC. In particular, lithography has been the most common technique performed. Nevertheless, this is a highly expensive process with complicated and time-consuming manual procedures performed in a clean-room environment [1][2][3][4][5][6]. In this sense, recent advances in microfabrication techniques, cell biology, microfluidics, and 3D printing enabled the rapid manufacturing of OoC along with biomimetic tissue micro-architectures, which can provide the basis for preclinical assays with greater predictive power [7]. Compared to conventional manufacturing techniques, 3D printing includes the advantages of unlimited design space, freedom of complex geometries, and reduction of waste products [8][9][10]. In particular, 3D bioprinting is an innovative and promising biofabrication strategy that has played an important role since it allows the deposition of biomaterial-encapsulated living cells in the manufacturing of complex 3D structures with high precision, high accuracy, and high throughput [11][12][13][14][15][16]. Considering those characteristics, bioprinters are expected to establish systems that mimic the microenvironment of the human body in a more appropriate way than the animal models and current 2D cell culture environments, enhancing the accuracy of the results and the clinical usage of OoC [13][17][18].
The aforementioned preclinical models used in biomedical investigation are outlined in Figure 1, highlighting the evolution of cell-culture models from simple two-dimensional to complex OoC platforms with 3D bioprinted models.
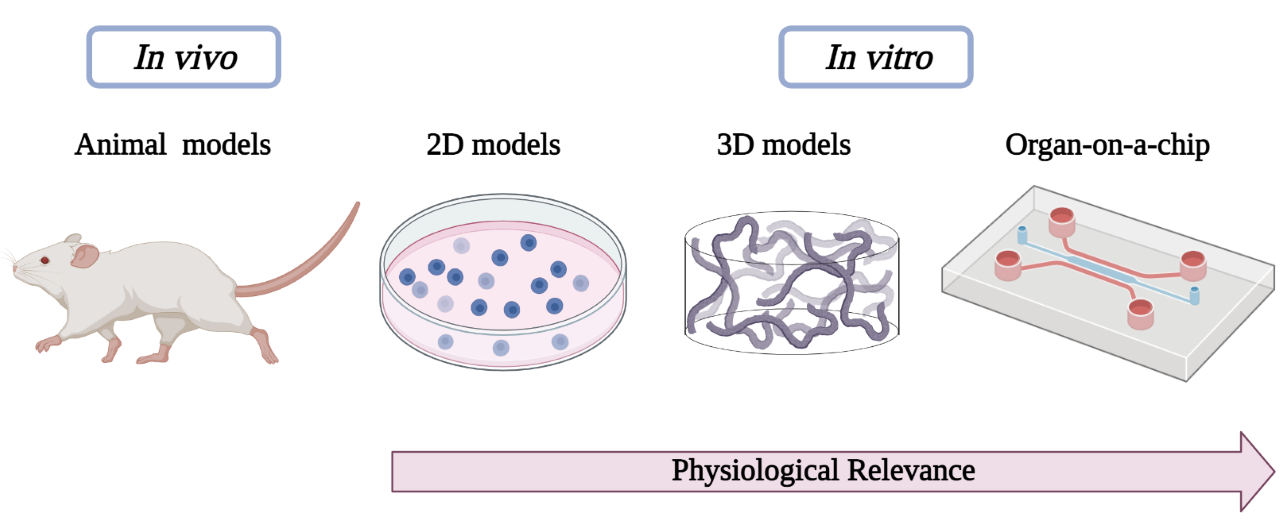
Figure 1. Schematic diagram showing preclinical models used in biomedical research.
2. 3D Printing Techniques and Their Applications to Organ-on-a-Chip Platforms
2.1. 3D Printing Techniques
3D printing has become a growing field in different areas and it has gained great interest, because of the ability to build complex structures through a layer-by-layer process with different materials in an affordable way [18]. For these reasons, some authors have been using 3D printing techniques to rapidly fabricate the microfluidic models and holders for OoC devices (Table 1).
Table 1. 3D printing techniques used to fabricate OoC platforms.
| Device | Printing Method | Application | Main Observations | Ref. | |
|---|---|---|---|---|---|
| Vessel-on-a-chip | - | Produce molds with diverse forms of channels. |  Reprinted with permission from ref. [19]. Copyright 2018 John Wiley and Sons. |
A simple and cytocompatible approach was developed for fabricating hydrogel-based user-defined chips, suitable for the growth of organ or vascularized tissue models. | [19] |
| Lung cancer-on-a-chip | Inkjet | 3D-printed chip holder and elastomeric microfluidic channels and microfluidic connectors for cell culture media routing on the higher part of the glass. | 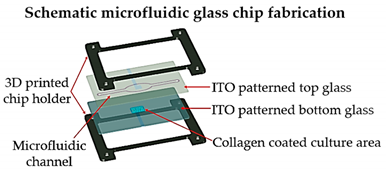 Reprinted with permission from ref. [20]. Copyright 2020 Elsevier. |
This lung cancer-on-chip system, includes integrated biosensors for real-time monitoring of physiological events, can be used with any organ tissue or monolayer micro-tumor models for on-chip toxicity studies. | [20] |
| Metastasis-on-a-Chip | Plaster-based 3D printing | 3D-printed inverted chamber/channel structures as molds. | 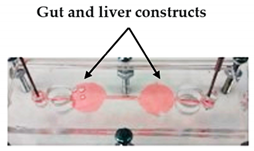 Reprinted with permission from ref. [21]. Copyright 2016 John Wiley and Sons. |
This system supports some aspects of the phenomena of metastasis, allowing to study the translocation of metastatic tumor cells from the primary tissue site to the downstream tissue site. | [21] |
| Vessel-on-a-chip | Extrusion-based 3D printing | 3D printing of channel prototypes with carbopol gel | 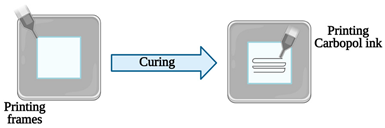 |
It is presented a highly affordable and practical approach in the manufacture of PDMS devices with closed fluid channels, which have great potential to reconstitute a human endothelium-on-a-chip | [2] |
| Kidney-on-a-chip | FDM | 3D-printed template for conventional soft lithography fabrication of PDMS-based OoC | 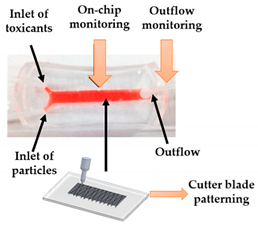 Reprinted with permission from ref. [22]. Copyright 2016 Elsevier. |
It is demonstrated the application of a 3D-printed template and a common cutter machine to provide a simple and affordable fabrication of OoC. | [22] |
| Multi-Organ-On-a-Chip | Laser SLA with epoxy resin | Produce master models for the chambers and channels of the fluidic device. | 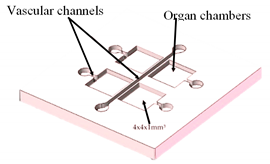 Reprinted from ref. [23]. |
This technology allows the design and rapid mass production of OoC devices. | [23] |
| Lung-on-a-chip | DLP | 3D-printed molds to manufacture a chip model with an open well design and with lower and upper layers to mimic the human lung. | 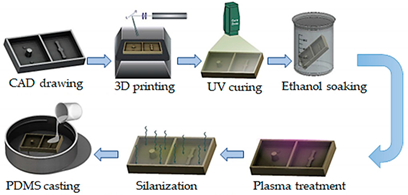 Reprinted from ref. [24]. Reprinted from ref. [24]. |
The fabrication technique allows the chip to be fabricated in less than a day, and the molds can also be utilized for repeated PDMS casting. Therefore, the technique is robust, cost-effective, and simple. | [24] |
SLA—stereolithography; FDM—fused deposition modelling; DLP—digital light processing.
The usage of 3D printing techniques to fabricate OoC is simple, cost-effective, robust, and allows the mass manufacture of customized OoC devices. However, attention must be taken in the selection of the 3D printing technique to obtain molds for PDMS casting. For example, molds printed via SLA/DLP methods may not be appropriate for PDMS casting because residual oligomers and monomers on the top of the 3D-printed pieces hamper PDMS polymerization [24]. Hence, the development of optimized surface treatments is crucial for ensuring long-term cell viability in OoC devices. Furthermore, the material utilized in 3D printing must be selected taking into account the curing temperature of the casting material in order to prevent material strain and microstructure deformation, which consequently can affect the cell viability.
2.2. 3D Bioprinting Techniques
As previously stated, 3D bioprinting can be described as the spatial distribution in a defined pattern of living cells. The cells are loaded and assembled through layer-by-layer deposition methods assisted by means of a computer, and used for the manufacture of organ analogs and living tissue for a different set of applications, such as pharmacokinetic, tissue engineering, cancer research, and regenerative medicine, among others [25]. For this purpose, biocompatible materials, such as alginate, gellan-gum, collagen, fibrin, and gelatin, are usually used to form hydrogels, called bioinks, to encapsulate cells (cf. Table 2) in order to protect them during the printing process.
Table 2. 3D bioprinting techniques used to fabricate OoC platforms.
| OoC Platform | Printing Method | Schematic Representation | Cells Types | Bioink | Ref. |
|---|---|---|---|---|---|
| Nervous System-on-a-Chip | Micro-extrusion 3D printing strategies | 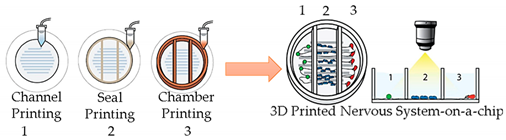 Reprinted with permission from ref. [26]. Copyright 2001 Royal Society of Chemistry. |
Schwann cells, superior cervical ganglia and hippocampal neurons and epithelial cells |
- | [26] |
| Central nervous system-on-a-chip | Magnetic bioprinting | 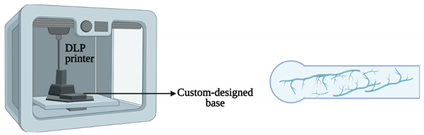 |
Spinal cord cells | Neural spheroids | [27] |
| Multi-tissue OoC with liver, heart and lung organoids | Microextrusion bioprinting | 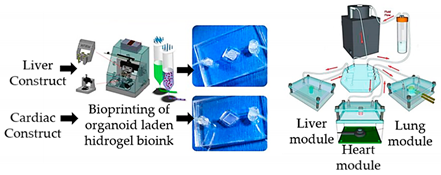 Reprinted from ref. [28]. |
Hepatocyte; stellate; Kupffer iPS; lung fibroblasts, epithelial, and endothelial cells. | Spherical organoids with HA-gelatin hydrogel (liver) and fibrin-gelatin bioink (cardiac). | [28] |
| 3D vascularized tissue-on-a-chip | Microextrusion bioprinting | 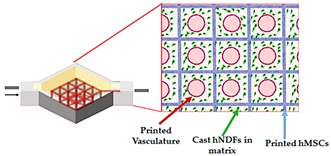 Reprinted from ref. [29]. |
hMSCs; hNDFs; HUVECs | Vascular ink (pluronic and thrombin) and cell-laden ink (gelatin–fibrin) | [29] |
| Liver-on-a-chip | Direct write bioprinter |
 |
HepG2/C3A cells | Hepatic spheroids and GelMA | [30] |
| Liver-on-a-chip | Microextrusion bioprinting | 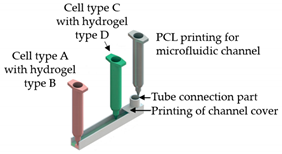 Reprinted from ref. [1]. |
HepG2; HUVECs. | Gelatin and liver dECM bioinks (collagen type 1) | [1] |
| Liver-on-a-chip | Microextrusion bioprinting | 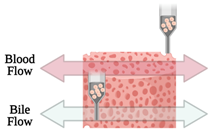 |
HepaRG and HUVECs | Gelatin and liver dECM bioinks (collagen type 1) | [31] |
| Liver Fibrosis-on-a-Chip | Microextrusion bioprinting | 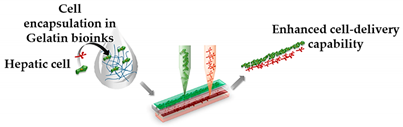 Reprinted with permission from ref. [32]. Copyright 2020 American Chemical Society. |
HepaRG, HUVECs and hepatic stellate cells |
Gelatin and liver dECM bioinks (collagen type 1) | [32] |
| Convoluted 3D renal proximal tubules-on-a-chip | Extrusion custom-designed, multi-material 3D bioprinter |  Reprinted from ref. [33]. |
PTECs-TERT1 | Two-part silicone elastomer; Pluronic and thrombin. |
[33] |
| Vessel-like structures-on-a-chip | Coaxial nozzle-assisted extrusion-based bioprinting | 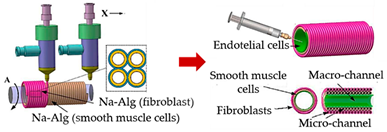 Reprinted with permission from ref. [34]. Copyright 2017 American Chemical Society |
L929 fibroblasts; endothelial cells and smooth muscle cells | Cell-laden alginate filaments | [34] |
| Vessel-on-a-chip | - |  |
HAECs; HASMC and NIH/3 T3 fibroblast cell lines | GelMA | [35] |
| Heart-on-a-Chip | Direct write bioprinter with a customized coaxial nozzle |
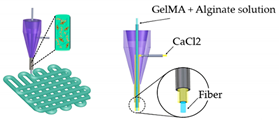 Reprinted with permission from ref. [36]. Copyright 2016 Elsevier |
HUVECs | Alginate-GelMA | [36] |
| Myocardium-on-a-chip | Extrusion-based 3D bioprinting | 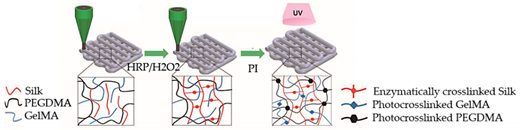 Reprinted with permission from ref. [37]. Copyright 2020 John Wiley and Sons. |
hiPSC-CSs | Non-mulberry silk-based ink GelMA and PEGDMA | [37] |
| Gut-on-a-chip | Dual cell-printing system supplemented with a core-shell nozzle | 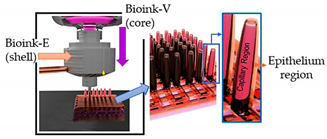 Reprinted with permission from ref. [38]. Copyright 2018 American Chemical Society. |
Caco-2 cells and HUVECs | Cell-laden collagen bioinks | [38] |
| Thrombosis-on-a-chip | Embedded extrusion bioprinting |  Reprinted with permission from ref. [39]. Copyright 2016 Royal Society of Chemistry. |
HUVECs | GelMA | [39] |
| Tumor array-on-a-chip | On-demand array printing | 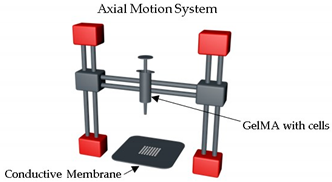 |
MDA-MB-231 breast tumor cells showed | GelMA | [40] |
| Placenta-on-a-chip | Extrusion-based 3D bioprinting | 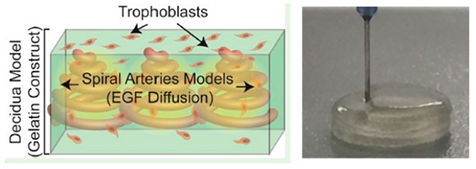 Reprinted with permission from ref. [41]. Copyright 2016 American Chemical Society. |
Human placental cell line and hMSCs | GelMA | [41] |
iPS—induced pluripotent stem cells; HA—hyaluronic acid; hMSCs—human mesenchymal stem cells; hNDFs—human neonatal dermal fibroblasts; HUVECs—human umbilical vein endothelial cells; HepG2—human hepatocellular carcinoma; HepaRG—terminally differentiated human hepatocellular carcinoma cells; dECM—decellularized extracellular matrix; PTECs—proximal tubule epithelial cells; TERT1—human telomerase reverse transcriptase; HAECs—primary human aortic endothelial cells; HASMC—human aortic smooth muscle cell line CRL1999; GelMA—gelatin methacryloyl; hiPSC-CSs—human-induced pluripotent stem cell-derived cardiac spheroids; PEGDMA—polyethylene glycol dimethacrylate; BMECs—human bone marrow endothelial cells.
It can be seen that 3D printing techniques are versatile and they can be applied to obtain a variety of OoC or multi-organ-on-a-chip, such as nervous-system-on-a chip, vascularized tissue-on-a-chip, liver-on-a-chip, renal tubule-on-a-chip, vessel-on-a-chip, myocardium-on-a-chip, gut-on-a-chip, thrombosis-on-a-chip, and tumor array-on-a-chip. These different cases are now presented.
2.3. New Approaches and Other Applications of 3D (Bio)Printing to Fabricate OoC Platforms without Specifying the Target Organ
Some researchers identified in the literature did not study OoC platforms by specifying the target organ; the results are summarized in Table 3.
Table 3. Novel approaches of 3D bioprinting/printing techniques suitable for the manufacturing of OoC platforms.
| 3D (Bio)Printing Technology | Schematic Representation | Ref. |
|---|---|---|
| Embedded extrusion bioprinting | 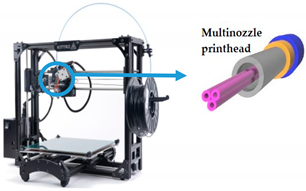 Reprinted from ref. [42]. |
[42] |
| Embedded extrusion bioprinting | 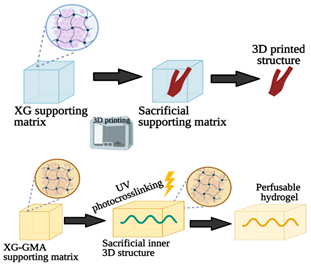 |
[43] |
| Embedded extrusion bioprinting | 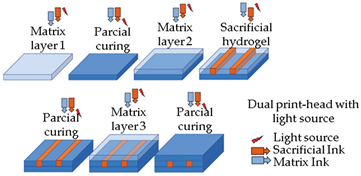 Reprinted with permission from ref. [44]. Copyright 2019 Elsevier. |
[44] |
| DLW and DLIP | 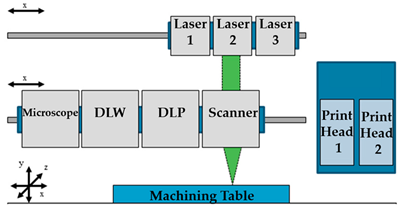 Reprinted from ref. [45]. |
[45] |
| LIFT printing | 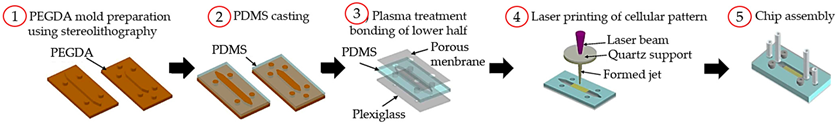 Reprinted with permission from ref. [46]. Copyright 2019 Royal Society of Chemistry. |
[46] |
| SLA and Bioprinting (BioScaffolder) | 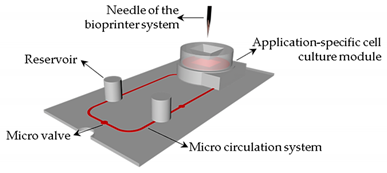 |
[47] |
DLW—direct laser writing; LIFT—laser-induced forward transfer; DLIP—direct laser interference patterning.
3. Other Challenges in Organs-on-Chip Devices: Sensors Integration
For a standard laboratory practice, OoC platforms require an accurate control and monitoring of the cell metabolism and environment, as well as of the biomarkers released by the organ models into the feeding medium. Currently, this monitoring is mainly achieved by off-line post-analysis, which besides being time-consuming is prone to contamination and sample degradation. To overcome this limitation, micro(bio)sensors have been investigated to be incorporated into those platforms to allow for real-time, robust, and autonomous monitoring of the organ models.
In the near future, multi-organ-on-a-chip and, ultimately, human-on-a-chip platforms are expected to be developed. For that purpose, a platform with integrated biosensors will be a huge step towards the advance of OoC platforms, providing physiological metabolism parameters of the organ model, as presented at Figure 4 [48]. In this way, innovative experimental studies will be possible and as a result it will help to improve our understanding about the evolution of certain pathologies and how they affect the overall system [48]. A more comprehensive review on this topic can be found elsewhere [49].
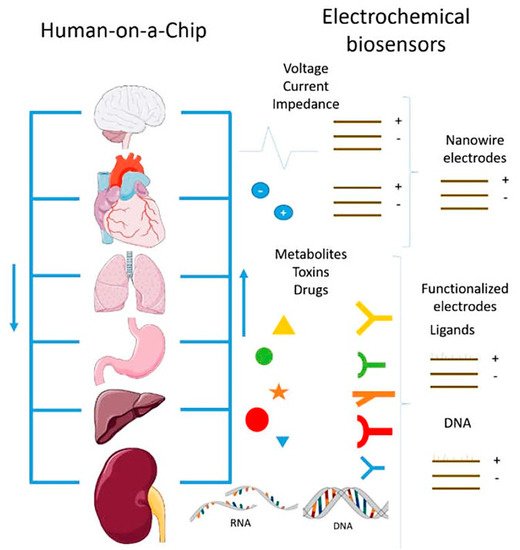
Figure 4. Representation of the biosensors for a human-on-a-chip platform. Reprinted from ref. [48].
4. Future Perspectives
Although great efforts for developing new and feasible 3D (bio)printing techniques have been made, wide-scale adoption and validation are still to be achieved. Through advances in 3D printing technologies, more physiologically relevant OoC models are expected and this will accelerate the commercialization of these models and their practical use in drug discovery to overcome several human diseases. Although the focus of this work lies in 3D (bio)printing, it should be mentioned that the variable “time” has also been integrated, giving rise to 4D bioprinting, where printed items (for example, responsive biocompatible materials or cells) are able to change their functionalities or shapes with time once an external stimulus is imposed [50].
References
- Lee, H.; Cho, D.W. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip 2016, 16, 2618–2625.
- Ozbolat, V.; Dey, M.; Ayan, B.; Ozbolat, I.T. Extrusion-based printing of sacrificial Carbopol ink for fabrication of microfluidic devices. Biofabrication 2019, 11.
- Probst, C.; Schneider, S.; Loskill, P. High-throughput organ-on-a-chip systems: Current status and remaining challenges. Curr. Opin. Biomed. Eng. 2018, 6, 33–41.
- Catarino, S.O.; Rodrigues, R.O.; Pinho, D.; Miranda, J.M.; Minas, G.; Lima, R. Blood Cells Separation and Sorting Techniques of Passive Microfluidic Devices: From Fabrication to Applications. Micromachines 2019, 10, 593.
- Chen, L.-J.; Kaji, H. Modeling angiogenesis with micro- and nanotechnology. Lab Chip 2017, 17, 4186–4219.
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical microfluidic devices by using low-cost fabrication techniques: A review. J. Biomech. 2016, 49, 2280–2292.
- Saglam-Metiner, P.; Gulce-Iz, S.; Biray-Avci, C. Bioengineering-inspired three-dimensional culture systems: Organoids to create tumor microenvironment. Gene 2019, 686, 203–212.
- Miri, A.K.; Mirzaee, I.; Hassan, S.; Oskui, M.; Nieto, D.; Shrike, Y. Effective bioprinting resolution in tissue model fabrication. Lab Chip 2019, 19, 2019–2037.
- Li, Y.; Mao, Q.; Li, X.; Yin, J.; Wang, Y.; Fu, J.; Huang, Y. High-fidelity and high-efficiency additive manufacturing using tunable pre-curing digital light processing. Addit. Manuf. 2019, 30, 100889.
- Miri, A.; Mostafavi, E.; Khorsandi, D.; Hu, S.-K.; Malpica, M.; Khademhosseini, A. Bioprinters for organs-on-chips. Int. Soc. Biofabr. 2019, 11, 042002.
- Ding, H.; Illsley, N.P.; Chang, R.C. 3D Bioprinted GelMA Based Models for the Study of Trophoblast Cell Invasion. Sci. Rep. 2019, 9.
- Matai, I.; Kaur, G.; Seyedsalehi, A.; Mcclinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2019, 2020, 119536.
- Reid, J.A.; Mollica, P.A.; Johnson, G.D.; Ogle, R.C.; Bruno, R.D.; Sachs, P.C. Accessible bioprinting: Adaptation of a low-cost 3D-printer for precise cell placement and stem cell differentiation. Biofabrication 2016, 8, 025017.
- Gungor-ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Dokmeci, M.R.; Sciences, N.; Angeles, L.; Arabia, S.; Angeles, L. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946.
- Ozbolat, I.T.; Moncal, K.K.; Gudapati, H. Evaluation of bioprinter technologies. Addit. Manuf. 2017, 13, 179–200.
- Ambhorkar, P.; Rakin, R.H.; Wang, Z.; Kumar, H.; Kim, K. Biofabrication strategies for engineering heterogeneous artificial tissues. Addit. Manuf. 2020, 36, 101459.
- Deo, K.A.; Singh, K.A.; Peak, C.W.; Alge, D.L.; Gaharwar, A.K. Bioprinting 101: Design, Fabrication, and Evaluation of Cell-Laden 3D Bioprinted Scaffolds. Tissue Eng. Part A 2020, 26, 318–338.
- Yi, H.; Lee, H.; Cho, D. 3D Printing of Organs-On-Chips. Bioengineering 2017, 4, 10.
- Nie, J.; Gao, Q.; Wang, Y.; Zeng, J.; Zhao, H.; Sun, Y.; Shen, J.; Ramezani, H.; Fu, Z.; Liu, Z.; et al. Vessel-on-a-chip with Hydrogel-based Microfluidics. Small 2018, 14, 1–14.
- Khalid, M.A.U.; Kim, Y.S.; Ali, M.; Lee, B.G.; Cho, Y.-J.; Choi, K.H. A lung cancer-on-chip platform with integrated biosensors for physiological monitoring and toxicity assessment. Biochem. Eng. J. 2020, 155, 107469.
- Skardal, A.; Devarasetty, M.; Forsythe, S.; Atala, A.; Soker, S. A reductionist metastasis-on-a-chip platform for in vitro tumor progression modeling and drug screening. Biotechnol. Bioeng. 2016, 113, 2020–2032.
- Cho, S.; Islas-Robles, A.; Nicolini, A.M.; Monks, T.J.; Yoon, J.-Y. In situ, dual-mode monitoring of organ-on-a-chip with smartphone-based fluorescence microscope. Biosens. Bioelectron. 2016, 86, 697–705.
- Lantada, A.D.; Pfleging, W.; Besser, H.; Guttmann, M.; Wissmann, M.; Plewa, K.; Smyrek, P.; Piotter, V.; García-Ruíz, J.P. Research on the methods for the mass production of multi-scale organs-on-chips. Polymers 2018, 10, 1238.
- Shrestha, J.; Ghadiri, M.; Shanmugavel, M.; Razavi Bazaz, S.; Vasilescu, S.; Ding, L.; Ebrahimi Warkiani, M. A rapidly prototyped lung-on-a-chip model using 3D-printed molds. Organs-Chip 2019, 1, 100001.
- Datta, P.; Ayan, B.; Ozbolat, I.T. Bioprinting for Vascular and Vascularized Tissue Biofabrication. Acta Biomater. 2017, 51, 1–20.
- Johnson, B.N.; Lancaster, K.Z.; Hogue, I.B.; Meng, F.; Kong, Y.L.; Enquist, L.W.; McAlpine, M.C. 3D printed nervous system on a chip. Lab Chip 2016, 16, 1393–1400.
- Bowser, D.A.; Moore, M.J. Biofabrication of neural microphysiological systems using magnetic spheroid bioprinting. Int. Soc. Biofabr. 2020, 12, 015002.
- Skardal, A.; Murphy, S.V.; Devarasetty, M.; Mead, I.; Kang, H.; Seol, Y.; Zhang, Y.S.; Shin, S.; Zhao, L.; Aleman, J.; et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci. Rep. 2017, 7, 8837.
- Kolesky, D.B.; Homan, K.A.; Skylar-scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184.
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101.
- Lee, H.; Chae, S.; Kim, J.Y.; Han, W.; Kim, J.; Choi, Y. Cell-printed 3D liver-on-a-chip possessing a liver microenvironment and biliary system. Int. Soc. Biofabr. 2019, 11, 025001.
- Lee, H.; Kim, J.; Choi, Y.; Cho, D.W. Application of Gelatin Bioinks and Cell-Printing Technology to Enhance Cell Delivery Capability for 3D Liver Fibrosis-on-a-Chip Development. ACS Biomater. Sci. Eng. 2020, 6, 2469–2477.
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 2016, 6, 34845.
- Gao, Q.; Liu, Z.; Lin, Z.; Qiu, J.; Liu, Y.; Liu, A.; Xiang, M.; Chen, B.; Fu, J.; He, Y. 3D Bioprinting of Vessel-like Structures with Multi-level Fluidic Channels. ACS Biomater. Sci. Eng. 2017.
- Abudupataer, M.; Chen, N.; Yan, S.; Alam, F.; Shi, Y.; Wang, L.; Lai, H.; Li, J.; Zhu, K.; Wang, C. Bioprinting a 3D vascular construct for engineering a vessel-on-a-chip. Biomed. Microdevices 2020, 22, 10.
- Zhang, Y.S.; Aleman, J.; Arneri, A.; Bersini, S.; Shin, S.-R.S.R.; Dokmeci, M.R.; Khademhosseini, A.; Arabia, S.; Zhu, K.; Goli-Malekabadi, Z.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59.
- Mehrotra, S.; De Melo, B.A.G.; Hirano, M.; Keung, W.; Li, R.A.; Mandal, B.B.; Shin, S.R. Nonmulberry Silk Based Ink for Fabricating Mechanically Robust Cardiac Patches and Endothelialized Myocardium-on-a-Chip Application. Adv. Mater. 2020, 30, 1907436.
- Kim, W.; Kim, G. Intestinal Villi Model with Blood Capillaries Fabricated Using Collagen-Based Bioink and Dual-Cell-Printing Process. ACS Appl. Mater. Interfaces 2018, 10, 41185–41196.
- Zhang, Y.S.; Davoudi, F.; Walch, P.; Manbachi, A.; Luo, X.; Dell’Erba, V.; Miri, A.K.; Albadawi, H.; Arneri, A.; Li, X.; et al. Bioprinted thrombosis-on-a-chip. Lab Chip 2016, 16, 4097–4105.
- Xie, M.; Gao, Q.; Fu, J.; Chen, Z.; He, Y. Bioprinting of novel 3D tumor array chip for drug screening. Bio-Design Manuf. 2020, 3, 175–188.
- Kuo, C.; Eranki, A.; Placone, J.K.; Rhodes, K.R.; Aranda-espinoza, H.; Fernandes, R.; Fisher, J.P.; Kim, P.C.W. Development of a 3D Printed, Bioengineered Placenta Model to Evaluate the Role of Trophoblast Migration in Preeclampsia. ACS Biomater. Sci. Eng. 2016, 2, 1817–1826.
- Rocca, M.; Fragasso, A.; Liu, W.; Heinrich, M.A.; Zhang, Y.S. Embedded Multimaterial Extrusion Bioprinting. SLAS Technol. 2018, 23, 154–163.
- Patrício, S.; Sousa, L.; Correia, T.; Gaspar, V.; Pires, L.; Oliveira, J.; Mano, J. Freeform 3D Printing using a Continuous Viscoelastic Supporting Matrix. Int. Soc. Biofabr. 2020, 12, 035017.
- Ji, S.; Almeida, E.; Guvendiren, M. 3D bioprinting of complex channels within cell-laden hydrogels. Acta Biomater. 2019, 95, 214–224.
- Günther, K.; Sonntag, F.; Lasagni, A.F.; Moritzer, E.; Hirsch, A.; Klotzbach, U.; Lasagni, A.F. Universal micromachining platform and basic technologies for the manufacture and marking of microphysiological systems. Micromachines 2017, 8, 246.
- Xiong, R.; Chai, W.; Huang, Y. Laser printing-enabled direct creation of cellular heterogeneity in lab-on-a-chip devices. Lab Chip 2019, 19, 1644–1656.
- Sonntag, F.; Schmieder, F.; Ströbel, J.; Grünzner, S.; Busek, M.; Günther, K.; Steege, T.; Polk, C.; Klotzbach, U. Universal lab-on-a-chip platform for complex, perfused 3D cell cultures. Microfluid. BioMEMS Med. Microsyst. XIV 2016, 9705, 970516.
- Sosa-Hernández, J.E.; Villalba-Rodríguez, A.M.; Romero-Castillo, K.D.; Aguilar-Aguila-Isaías, M.A.; García-Reyes, I.E.; Hernández-Antonio, A.; Ahmed, I.; Sharma, A.; Parra-Saldívar, R.; Iqbal, H.M.N. Organs-on-a-chip module: A review from the development and applications perspective. Micromachines 2018, 9, 536.
- Rodrigues, R.O.; Sousa, P.C.; Gaspar, J.; Bañobre-López, M.; Lima, R.; Minas, G. Organ-on-a-Chip: A Preclinical Microfluidic Platform for the Progress of Nanomedicine. Small 2020, 16, 2003517.
- Mitchell, A.; Lafont, U.; Hołyńska, M.; Semprimoschnig, C. Additive manufacturing—A review of 4D printing and future applications. Addit. Manuf. 2018, 24, 606–626.




