Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christiane Funk | + 2156 word(s) | 2156 | 2021-06-09 05:44:34 | | | |
| 2 | Lily Guo | -2 word(s) | 2154 | 2021-06-15 04:08:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Funk, C.; Mishra, L. FtsHi Enzymes of Arabidopsis thaliana. Encyclopedia. Available online: https://encyclopedia.pub/entry/10679 (accessed on 08 February 2026).
Funk C, Mishra L. FtsHi Enzymes of Arabidopsis thaliana. Encyclopedia. Available at: https://encyclopedia.pub/entry/10679. Accessed February 08, 2026.
Funk, Christiane, Laxmi Mishra. "FtsHi Enzymes of Arabidopsis thaliana" Encyclopedia, https://encyclopedia.pub/entry/10679 (accessed February 08, 2026).
Funk, C., & Mishra, L. (2021, June 09). FtsHi Enzymes of Arabidopsis thaliana. In Encyclopedia. https://encyclopedia.pub/entry/10679
Funk, Christiane and Laxmi Mishra. "FtsHi Enzymes of Arabidopsis thaliana." Encyclopedia. Web. 09 June, 2021.
Copy Citation
FtsH metalloproteases found in eubacteria, animals, and plants are well-known for their vital role in the maintenance and proteolysis of membrane proteins. Their location is restricted to organelles of endosymbiotic origin, the chloroplasts, and mitochondria. In the model organism Arabidopsis thaliana, there are 17 membrane-bound FtsH proteases containing an AAA+ (ATPase associated with various cellular activities) and a Zn2+ metalloprotease domain.
AAA-type protease
FtsH metalloprotease
chloroplast
embryo lethal
leaf variegation
plastid biogenesis
protein import
oxidative stress
1. Introduction
Cells have evolved an extensive system of molecular chaperones, folding catalysts, and proteases that control protein quality and prevent damage. In addition to the well-studied degradative removal of damaged or superfluous proteins [1], proteolysis is highly important in regulating protein preprocessing, maturation, post-translational protein modifications, and signaling [2][3]. Therefore, it is is no overstatement that proteolysis is directly or indirectly involved in most cellular processes [4].
1.1. Proteases in the Plant Chloroplast
Plant proteases associated with a particular proteolytic activity are present in various cellular compartments and organelles constituting up to 3% of the plant proteome [5][6]. Proteases are classified according to their catalytic types. Except for glutamic acid proteases, representatives from all protease classes (threonine, cysteine, serine, aspartic, metalloproteases) have been detected in the plant Arabidopsis thaliana [7]. The chloroplast is a unique organelle of the plant cell; absorption and conversion of light energy in the photosynthetic reaction lead to a permanent need for protein turnover (processing and degradation) to adapt to different light conditions. Excess light adsorption further can cause the formation of reactive oxygen species and damage proteins. Therefore, protein quality and quantity controls are essential [8][9]. In addition to photosynthesis, several metabolic reactions happen in the chloroplast, including the biosynthesis of lipids, amino acids, chlorophylls, and carotenoids; therefore, plastidic proteases are vital regulators [9]. More than 20 different families of chloroplast proteases have been detected, with members localized in specific sub-organellar compartments [3][9].
1.2. Plant Pseudo-Proteases
In addition to the proteolytically active proteases, members with mutations in their active site attracted the attention of researchers. Despite their putative proteolytically inactivity, many of these pseudo-proteases have essential roles in the cell, ranging from structural proteins via chaperones [10][11] to enzymes with a new function [12][13][14][15][16][17][18][19][20]. In the chloroplast, pseudo-proteases are found in the families of serine Clp-proteases (ClpRs, [21][22][23]) and FtsH metalloproteases (FtsHis [18][24][25][26]). ClpRs of the Clp protease family lack their catalytic triad. These proteolytically inactive subunits are of structural importance to form a tetradecameric proteolytic core together with the catalytically active ClpP subunits [21][22][23]. Most ClpR proteins are essential for the proteolytic function of the Clp core function.
This review will focus on pseudo-proteases belonging to the family of the membrane-bound ATP-dependent FtsH metalloproteases, which are termed FtsHi (i-inactive) [23]. These presumably proteolytically inactive FtsHi enzymes are restricted to the plant chloroplast and, similar to the ClpR, affect chloroplast and overall plant development [17][23][24][25].
2. Filamentation Temperature-Sensitive Protein H (FtsH) Protease Family
The name FtsH (filamentation temperature-sensitive) erroneously originated from the growth behavior of a Y16 Escherichia coli strain deficient in its ftsh gene. However, later, a second, independent mutation was found to be responsible for the observed temperature-sensitive phenotype [23].
FtsH proteases, known as zincins, belong to the MEROPS peptidase family M41, which in turn belongs to a larger family of zinc metalloproteases [27]. Within the M41 peptidase domain, the Zn2+ ion is ligated by two histidine residues, forming the HEXXH motif (where X is any uncharged residue) as well as a glutamic acid residue [28]. Functional homo- or hetero-hexameric complexes are inserted into the membrane by one or more N-terminal transmembrane domains per subunit [29][30][31][32]. The highly conserved AAA+ domain, a cassette of about 200–250 residues that contains the ATP-binding motif (Walker A and Walker B) and the second region of homology (SRH), is situated between the transmembrane region and the active site (Figure 1). Unlike other well-studied ATP-dependent proteases, FtsHs lack robust unfoldase activity [33]. Instead, ATP hydrolysis by FtsH is used to translocate unfolded substrates sequentially into the hexameric pore [33][34]. The AAA+ domain is required for nucleotide binding and hydrolysis [29][35] and responsible for alternating between a closed and open state of the FGV pore motif, which is a conserved hydrophobic area at the proteolytic chamber [29]. The substrate is pulled into the degradation chamber via a narrow pore [36][37]. Recent cryo-electron microscopy studies enabled the study of substrate processing of AAA+ proteins in detail (reviewed in [38]) and revealed a conserved spiraling organization of ATPase hexamers around the translocating protein substrate.
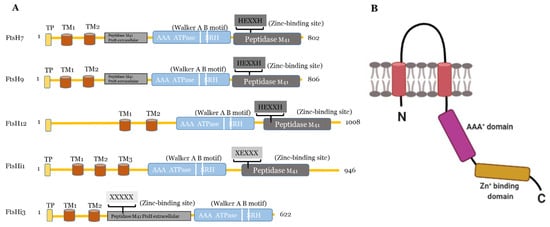
Figure 1. (A) Predicted domains and motifs of AtFtsHis in comparison to the presumably active AtFtsH7, 9, and 12. TP, transit peptide; transmembrane domains, TM1-3; Walker A B motifs are indicated as two white lines between the AAA+ ATPase and SRH; SRH, second region of homology. Active FtsH proteases contain the Zn2+-binding motif (HEXXH) in the their peptidase M41 domain, which is substituted or absent in presumably inactive FtsHis (substitution of both histidines indicated as XEXXX). In FtsHi3, the peptidase M41 domain is annotated as “FtsH extracellular” and is located at the N-terminal to the ATPase; its HEXXH motif is completely missing (XXXXX). FtsH7 and FtsH9 contain an FtsH extracellular” peptidase domain additionally to their protease domain. AtFtsHis are predicted to contain three (FtsHi1, 5), two (FtsHi2, 3), or one (FtsHi4) transmembrane domains (http://www.cbs.dtu.dk/services/TMHMM/, accessed on 3 April 2021) (Supplementary Figure S1 [26]). (B) Schematic drawing of the structure of a monomeric FtsH protease with two membrane-spanning regions (shown in red), the proteolytic domain (in range) and the AAA+-domain (in pink). Created with BioRender.com (accessed on 27 May 2021).
2.1. The FtsH Protease Family of Arabidopsis thaliana
The annual plant Arabidopsis thaliana contains 17 different FtsH proteases. Gene comparison studies showed that of the 12 FTSH genes potentially coding for fully functional proteases, ten are found in highly homologous pairs. While the pairs AtFtsH1/5, AtFtsH2/8, and AtFtsH7/9 are targeted to the chloroplast, AtFtsH3/10 and AtFtsH4 have been identified in mitochondria. AtFtsH11, the pair partner of AtFtsH4, was initially reported to be dual targeted to mitochondria and the chloroplast [39]. However, Wagner and coworkers confirmed its location to be only in chloroplasts [40]. AtFtsH3 and AtFtsH10 were shown not to be crucial for growth under optimal conditions [41]. Loss of AtFtsH4 leads to oxidative stress and the accumulation of oxidized proteins [42][43]. FtsH10 is involved in the assembly and/or stability of complexes I and V of the mitochondrial oxidative phosphorylation system [43].
2.1.1. FtsH Proteases Located in the Thylakoid Membrane
Of the plastidic FtsHs, FtsH1, 2, 5, and 8 are localized in the thylakoid membrane. These members are the most abundant FtsH proteases and extensively studied [44]; they form hetero-hexameric complexes, in which FtsH1 and FtsH5 (Type A) and FtsH2 and FtsH8 (Type B) can partially substitute for each other [45]. A threshold in the amount of type A and B subunits was postulated to determine the proper function and development of chloroplasts and thylakoid membrane [46][47][48][49]. This thylakoid located protease complex plays a vital role in the degradation and assembly of the Photosystem II reaction center protein D1 and other transmembrane subunits of the photosynthetic machinery. Mutants lacking FtsH2/VAR2 or FtsH5/VAR1 show strongly or slightly variegated leaves, respectively [50][51]. Functional loss of, e.g., FtsH2 results in upregulation of other FtsH proteins in the green leaf sectors to maintain proper function and development of the chloroplasts [47][50][52]. FtsH6 is also localized in the thylakoid membrane. It is essential for thermotolerance and thermomemory in seedlings [53], while no phenotype was observed in adult plants when grown in semi-natural outdoor conditions [54].
2.1.2. FtsH Proteases Located in the Chloroplast Envelope
The other plastidic FtsH enzymes are believed to be localized in the chloroplast envelope [19]. Deleting FtsH7 and 9 does not result in any obvious phenotype [53], and the proteases are not required for PSII repair [55]. FtsH11 is crucial for growth in long photoperiods [40] and thermotolerance [56][57]. FtsH12 was co-immuno-precipitated in a complex with FtsHi1, 2, 4, 5 and NAD-dependent malate dehydrogenase (MDH) and shown to be involved in protein import [58][59]. In addition to FtsHi1, 2, 4, and 5, even FtsHi3 belongs to the five plastidic FtsH homologues, which are incapable of proteolysis in Arabidopsis. The FtsHi enzymes either have a mutation in their HEXXH motif (FtsHi1, 2, 4, and 5), or the entire motif is missing (FtsHi3) [18][26]. Compared to AtFtsHi1, 2, 4, and 5, FtsHi3 contains a very short C-terminal domain. Interestingly, FtsHi3 also has undergone a domain swap: the whole M41 domain is located at the N-terminal instead of at the C-terminal to the AAA+ domain [26]. Comparing the domain organization of AtFtsHi3 with AtFtsHi1, AtFtsH7, 9, 12 (Figure 1), also AtFtsH7/9 contain this “peptidase M41 FtsH extracellular” domain N-terminal to the AAA+ domain, which is additional to their protease domain located in the C-terminal to the AAA+ domain. This additional domain is not present in other FtsHs or FtsHis. Whether the N-terminal “peptidase M41 FtsH extracellular” domain of FtsH7, FtsH9, and FtsHi3 enables these enzymes to form a common complex with a specific function remains to be shown. Three independent pre-protein translocating models (pSSU-TEV-protein A, pL11Flag-TEV-Protein A, pLHCP-TEV-protein A) suggested FtsHi3 to form a 1-MD complex separate from the FtsH12/FtsHi1,2,4,5/MDH complex [58] and different to the 1-MD TIC complex [60]. The identity of other components in this complex is unknown. FTSHi3 is not co-expressed with the tight cluster of FTSH12/FTSHi1, 2, 4, 5, but instead with a gene encoding OTP51 [26][58][59]. This pentatricopeptide repeat protein is required for the splicing of group IIa introns and impacts photosystem I and II assembly [61]. The tight co-expression with FTSHi3 indicates a common function of OTP51 and FtsHi3; therefore, OTP51 is another hypothetical complex partner.
2.1.2. FtsH Proteases Located in the Chloroplast Envelope
The other plastidic FtsH enzymes are believed to be localized in the chloroplast envelope [19]. Deleting FtsH7 and 9 does not result in any obvious phenotype [53], and the proteases are not required for PSII repair [55]. FtsH11 is crucial for growth in long photoperiods [40] and thermotolerance [56][57]. FtsH12 was co-immuno-precipitated in a complex with FtsHi1, 2, 4, 5 and NAD-dependent malate dehydrogenase (MDH) and shown to be involved in protein import [58][59]. In addition to FtsHi1, 2, 4, and 5, even FtsHi3 belongs to the five plastidic FtsH homologues, which are incapable of proteolysis in Arabidopsis. The FtsHi enzymes either have a mutation in their HEXXH motif (FtsHi1, 2, 4, and 5), or the entire motif is missing (FtsHi3) [18][26]. Compared to AtFtsHi1, 2, 4, and 5, FtsHi3 contains a very short C-terminal domain. Interestingly, FtsHi3 also has undergone a domain swap: the whole M41 domain is located at the N-terminal instead of at the C-terminal to the AAA+ domain [26]. Comparing the domain organization of AtFtsHi3 with AtFtsHi1, AtFtsH7, 9, 12 (Figure 1), also AtFtsH7/9 contain this “peptidase M41 FtsH extracellular” domain N-terminal to the AAA+ domain, which is additional to their protease domain located in the C-terminal to the AAA+ domain. This additional domain is not present in other FtsHs or FtsHis. Whether the N-terminal “peptidase M41 FtsH extracellular” domain of FtsH7, FtsH9, and FtsHi3 enables these enzymes to form a common complex with a specific function remains to be shown. Three independent pre-protein translocating models (pSSU-TEV-protein A, pL11Flag-TEV-Protein A, pLHCP-TEV-protein A) suggested FtsHi3 to form a 1-MD complex separate from the FtsH12/FtsHi1,2,4,5/MDH complex [58] and different to the 1-MD TIC complex [60]. The identity of other components in this complex is unknown. FTSHi3 is not co-expressed with the tight cluster of FTSH12/FTSHi1, 2, 4, 5, but instead with a gene encoding OTP51 [26][58][59]. This pentatricopeptide repeat protein is required for the splicing of group IIa introns and impacts photosystem I and II assembly [61]. The tight co-expression with FTSHi3 indicates a common function of OTP51 and FtsHi3; therefore, OTP51 is another hypothetical complex partner.
3. Pseudo-Proteases with an Important Enzymatic Activity
In addition to being pseudo-proteases, the AAA+ domain of FtsHis is intact and—based on the seed lethality of many FtsHi mutants—highly important for their activity, plastid, and overall plant development. Four out of the five AtFtsHi members have been demonstrated to form an inner envelope-bound heteromeric AAA+ (ATPase associated with diverse cellular activities) ATPase complex. This complex consisting of FtsH12/FtsHi1,2,4,5/pdNAD-MDH was found to be involved in ATP-driven protein import across the chloroplast envelope [58][59][62][63]. Even Ycf2 was observed being part of this 2 MDa complex using transgenic lines overexpressing FTSH12 [58]. Still, the protein could not be detected in complexes isolated from wild-type and tic56-3 plastids using a combination of native gel electrophoresis and protein quantification [64] and neither after pull-down of FtsH12 using its native promoter [65]. Kikuchi and coworkers [57] determined the super-complex Ycf2-FtsH12-FtsHi1,2,4,5-pdNAD-MDH physically to interact with TIC components such as Tic214 (Ycf1), Tic100, and Tic56 as well as with the pre-protein translocation components Toc75 and Toc159 [59]. In this complex, neither pdNAD-MDH activity [59] nor FtsH12 proteolytic activity [57] are required; an FtsH12 (H769Y) mutant developed normal chloroplasts with functional pre-protein import abilities.
The finding of ATP-driven protein import across the chloroplast membrane by an FtsH12/FtsHi1,2,4,5/pdNAD-MDH complex has steered an intense debate [66][67] on the importance of the long-accepted chloroplast protein import machinery, i.e., Tic110 and Tic40 forming a common translocon in the inner chloroplast membrane and recruiting the stromal chaperones Hsp93/ClpC1, cpHsp70, and Hsp90C [67]. If indeed the FtsH12/FtsHi complex plays the main role in protein import into the chloroplast [67] remains to be shown. The absence of this complex in most monocots as well as its lower impact in adult plants rather point to a specific function during chloroplast development in dicotyledons. We refer th
References
- Ehrmann, M.; Clausen, T. Proteolysis as a Regulatory Mechanism. Annu. Rev. Genet. 2004, 38, 709–724.
- Stael, S.; Van Breusegem, F.; Gevaert, K.; Nowack, M.K. Plant Proteases and Programmed Cell Death; Oxford University Press: Oxford, UK, 2019.
- van Wijk, K.J. Protein Maturation and Proteolysis in Plant Plastids, Mitochondria, and Peroxisomes. Annu. Rev. Plant Biol. 2015, 66, 75–111.
- López-Otín, C.; Bond, J.S. Proteases: Multifunctional enzymes in life and disease. J. Biol. Chem. 2008, 283, 30433–30437.
- Bonner, P.L. Peptidases in Plant Tissue; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 1–12.
- García-Lorenzo, M.; Sjödin, A.; Jansson, S.; Funk, C. Protease gene families in Populus and Arabidopsis. BMC Plant Biol. 2006, 6, 30.
- Schaller, A. A cut above the rest: The regulatory function of plant proteases. Planta 2004, 220, 183–197.
- Adam, Z. Chloroplast proteases and their role in photosynthesis regulation. In Regulation of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 2001; pp. 265–276.
- Nishimura, K.; Kato, Y.; Sakamoto, W. Chloroplast proteases: Updates on proteolysis within and across suborganellar compartments. Plant Physiol. 2016, 171, 2280–2293.
- Rep, M.; van Dijl, J.M.; Suda, K.; Schatz, G.; Grivell, L.A.; Suzuki, C.K. Promotion of mitochondrial membrane complex assembly by a proteolytically inactive yeast Lon. Science 1996, 274, 103–106.
- Voos, W.; Pollecker, K. The mitochondrial Lon protease: Novel functions off the beaten track? Biomolecules 2020, 10, 253.
- Zhou, J.W.; Fang, L.R.; Yang, Z.X.; Xu, S.G.; Lv, M.T.; Sun, Z.; Chen, J.Y.; Wang, D.; Gao, J.; Xiao, S.B. Identification of novel proteolytically inactive mutations in coronavirus 3C-like protease using a combined approach. FASEB J. 2019, 33, 14575–14587.
- Klemenčič, M.; Asplund-Samuelsson, J.; Dolinar, M.; Funk, C. Phylogenetic distribution and diversity of bacterial pseudo-orthocaspases underline their putative role in photosynthesis. Front. Plant Sci. 2019, 10, 293.
- Reynolds, S.L.; Fischer, K. Pseudoproteases: Mechanisms and function. Biochem. J. 2015, 468, 17–24.
- Malnoe, A.; Wang, F.; Girard-Bascou, J.; Wollman, F.A.; de Vitry, C. Thylakoid FtsH protease contributes to photosystem II and cytochrome b6f remodeling in Chlamydomonas reinhardtii under stress conditions. Plant Cell 2014, 26, 373–390.
- Pulido, P.; Toledo-Ortiz, G.; Phillips, M.A.; Wright, L.P.; Rodriguez-Concepcion, M. Arabidopsis J-protein J20 delivers the first enzyme of the plastidial isoprenoid pathway to protein quality control. Plant Cell 2013, 25, 4183–4194.
- Pulido, P.; Llamas, E.; Llorente, B.; Ventura, S.; Wright, L.P.; Rodriguez-Concepcion, M. Specific Hsp100 Chaperones Determine the Fate of the First Enzyme of the Plastidial Isoprenoid Pathway for Either Refolding or Degradation by the Stromal Clp Protease in Arabidopsis. PLoS Genet. 2016, 12, e1005824.
- Wagner, R.; Aigner, H.; Funk, C. FtsH proteases located in the plant chloroplast. Physiol. Plant. 2012, 145, 203–214.
- Ferro, M.; Brugiere, S.; Salvi, D.; Seigneurin-Berny, D.; Court, M.; Moyet, L.; Ramus, C.; Miras, S.; Mellal, M.; Le Gall, S.; et al. AT_CHLORO, a comprehensive chloroplast proteome database with subplastidial localization and curated information on envelope proteins. Mol. Cell Proteom. 2010, 9, 1063–1084.
- Wu, J.; Jin, Y.; Zhong, S.; Chen, R.; Zhu, S.; Wang, W.; Lu, Q.; Xiong, Y. A unique group of inactive serine protease homologues from snake venom. Toxicon 2008, 52, 277–284.
- Nishimura, K.; Apitz, J.; Friso, G.; Kim, J.; Ponnala, L.; Grimm, B.; van Wijk, K.J. Discovery of a Unique Clp Component, ClpF, in Chloroplasts: A Proposed Binary ClpF-ClpS1 Adaptor Complex Functions in Substrate Recognition and Delivery. Plant Cell 2015, 27, 2677–2691.
- Nishimura, K.; van Wijk, K.J. Organization, function and substrates of the essential Clp protease system in plastids. Biochim. Biophys. Acta 2015, 1847, 915–930.
- Andersson, F.I.; Tryggvesson, A.; Sharon, M.; Diemand, A.V.; Classen, M.; Best, C.; Schmidt, R.; Schelin, J.; Stanne, T.M.; Bukau, B.; et al. Structure and function of a novel type of ATP-dependent Clp protease. J. Biol. Chem. 2009, 284, 13519–13532.
- Sokolenko, A.; Pojidaeva, E.; Zinchenko, V.; Panichkin, V.; Glaser, V.M.; Herrmann, R.G.; Shestakov, S.V. The gene complement for proteolysis in the cyanobacterium Synechocystis sp. PCC 6803 and Arabidopsis thaliana chloroplasts. Curr. Genet. 2002, 41, 291–310.
- Janska, H.; Kwasniak, M.; Szczepanowska, J. Protein quality control in organelles—AAA/FtsH story. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 381–387.
- Mishra, L.S.; Mielke, K.; Wagner, R.; Funk, C. Reduced expression of the proteolytically inactive FtsH members has impacts on the Darwinian fitness of Arabidopsis thaliana. J. Exp. Bot. 2019, 70, 2173–2184.
- Rawlings, N.D.; Waller, M.; Barrett, A.J.; Bateman, A. MEROPS: The database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2013, 42, D503–D509.
- Saikawa, N.; Ito, K.; Akiyama, Y. Identification of glutamic acid 479 as the gluzincin coordinator of zinc in FtsH (HflB). Biochemistry 2002, 41, 1861–1868.
- Bieniossek, C.; Niederhauser, B.; Baumann, U.M. The crystal structure of apo-FtsH reveals domain movements necessary for substrate unfolding and translocation. Proc. Natl. Acad. Sci. USA 2009, 106, 21579–21584.
- Bieniossek, C.; Schalch, T.; Bumann, M.; Meister, M.; Meier, R.; Baumann, U. The molecular architecture of the metalloprotease FtsH. Proc. Natl. Acad. Sci. USA 2006, 103, 3066–3071.
- Ogura, T.; Wilkinson, A.J. AAA+ superfamily ATPases: Common structure—Diverse function. Genes Cells 2001, 6, 575–597.
- Tomoyasu, T.; Gamer, J.; Bukau, B.; Kanemori, M.; Mori, H.; Rutman, A.J.; Oppenheim, A.B.; Yura, T.; Yamanaka, K.; Niki, H.; et al. Escherichia-Coli Ftsh Is a Membrane-Bound, Atp-Dependent Protease Which Degrades the Heat-Shock Transcription Factor Sigma(32). EMBO J. 1995, 14, 2551–2560.
- Herman, C.; Prakash, S.; Lu, C.Z.; Matouschek, A.; Gross, C.A. Lack of a robust unfoldase activity confers a unique level of substrate specificity to the universal AAA protease FtsH. Mol. Cell 2003, 11, 659–669.
- Asahara, Y.; Atsuta, K.; Motohashi, K.; Taguchi, H.; Yohda, M.; Yoshida, M. FtsH recognizes proteins with unfolded structure and hydrolyzes the carboxyl side of hydrophobic residues. J. Biochem. 2000, 127, 931–937.
- Karata, K.; Inagawa, T.; Wilkinson, A.J.; Tatsuta, T.; Ogura, T. Dissecting the role of a conserved motif (the second region of homology) in the AAA family of ATPases. Site-directed mutagenesis of the ATP-dependent protease FtsH. J. Biol. Chem. 1999, 274, 26225–26232.
- Hinnerwisch, J.; Fenton, W.A.; Furtak, K.J.; Farr, G.W.; Horwich, A.L. Loops in the central channel of ClpA chaperone mediate protein binding, unfolding, and translocation. Cell 2005, 121, 1029–1041.
- Park, E.; Rho, Y.M.; Koh, O.J.; Ahn, S.W.; Seong, I.S.; Song, J.J.; Bang, O.; Seol, J.H.; Wang, J.; Eom, S.H.; et al. Role of the GYVG pore motif of HslU ATPase in protein unfolding and translocation for degradation by HslV peptidase. J. Biol. Chem. 2005, 280, 22892–22898.
- Puchades, C.; Sandate, C.R.; Lander, G.C. The molecular principles governing the activity and functional diversity of AAA+ proteins. Nat. Rev. Mol. Cell Biol. 2020, 21, 43–58.
- Urantowka, A.; Knorpp, C.; Olczak, T.; Kolodziejczak, M.; Janska, H. Plant mitochondria contain at least two i-AAA-like complexes. Plant Mol. Biol. 2005, 59, 239–252.
- Wagner, R.; Von Sydow, L.; Aigner, H.; Netotea, S.; Brugière, S.; Sjögren, L.; Ferro, M.; Clarke, A.; Funk, C. Deletion of FtsH11 protease has impact on chloroplast structure and function in Arabidopsis thaliana when grown under continuous light. Plant Cell Environ. 2016, 39, 2530–2544.
- Kolodziejczak, M.; Skibior-Blaszczyk, R.; Janska, H. m-AAA Complexes Are Not Crucial for the Survival of Arabidopsis under Optimal Growth Conditions Despite Their Importance for Mitochondrial Translation. Plant Cell Physiol. 2018, 59, 1006–1016.
- Gibala, M.; Kicia, M.; Sakamoto, W.; Gola, E.M.; Kubrakiewicz, J.; Smakowska, E.; Janska, H. The lack of mitochondrial AtFtsH4 protease alters Arabidopsis leaf morphology at the late stage of rosette development under short-day photoperiod. Plant J. 2009, 59, 685–699.
- Marta, K.; Marta, G.; Adam, U.; Hanna, J. The significance of Arabidopsis AAA proteases for activity and assembly/stability of mitochondrial OXPHOS complexes. Physiol. Plant. 2007, 129, 135–142.
- Kato, Y.; Sakamoto, W. FtsH Protease in the Thylakoid Membrane: Physiological Functions and the Regulation of Protease Activity. Front. Plant Sci. 2018, 9, 855.
- Zaltsman, A.; Ori, N.; Adam, Z. Two types of FtsH protease subunits are required for chloroplast biogenesis and Photosystem II repair in Arabidopsis. Plant Cell 2005, 17, 2782–2790.
- Chen, M.; Choi, Y.D.; Voytas, D.F.; Rodermel, S. Mutations in the Arabidopsis VAR2 locus cause leaf variegation due to the loss of a chloroplast FtsH protease. Plant J. 2000, 22, 303–313.
- Miura, E.; Kato, Y.; Matsushima, R.; Albrecht, V.; Laalami, S.; Sakamoto, W. The balance between protein synthesis and degradation in chloroplasts determines leaf variegation in Arabidopsis yellow variegated mutants. Plant Cell 2007, 19, 1313–1328.
- Takechi, K.; Sodmergen; Murata, M.; Motoyoshi, F.; Sakamoto, W. The Yellow Variegated (VAR2) locus encodes a homologue of FtsH, an ATP-dependent protease in Arabidopsis. Plant Cell Physiol. 2000, 41, 1334–1346.
- Yu, F.; Liu, X.; Alsheikh, M.; Park, S.; Rodermel, S. Mutations in Suppressor of Variegation1, a factor required for normal chloroplast translation, suppress var2-mediated leaf variegation in Arabidopsis. Plant Cell 2008, 20, 1786–1804.
- Yu, F.; Park, S.; Rodermel, S.R. The Arabidopsis FtsH metalloprotease gene family: Interchangeability of subunits in chloroplast oligomeric complexes. Plant J. 2004, 37, 864–876.
- Sakamoto, W.; Zaltsman, A.; Adam, Z.; Takahashi, Y. Coordinated regulation and complex formation of yellow variegated1 and yellow variegated2, chloroplastic FtsH metalloproteases involved in the repair cycle of photosystem II in Arabidopsis thylakoid membranes. Plant Cell 2003, 15, 2843–2855.
- Zaltsman, A.; Feder, A.; Adam, Z. Developmental and light effects on the accumulation of FtsH protease in Arabidopsis chloroplasts—Implications for thylakoid formation and photosystem II maintenance. Plant J. 2005, 42, 609–617.
- Sedaghatmehr, M.; Mueller-Roeber, B.; Balazadeh, S. The plastid metalloprotease FtsH6 and small heat shock protein HSP21 jointly regulate thermomemory in Arabidopsis. Nat. Commun. 2016, 7, 12439.
- Wagner, R.; Aigner, H.; Pruzinska, A.; Jankanpaa, H.J.; Jansson, S.; Funk, C. Fitness analyses of Arabidopsis thaliana mutants depleted of FtsH metalloproteases and characterization of three FtsH6 deletion mutants exposed to high light stress, senescence and chilling. New Phytol. 2011, 191, 449–458.
- Shao, S.; Cardona, T.; Nixon, P.J. Early emergence of the FtsH proteases involved in photosystem II repair. Photosynthetica 2018, 56, 163–177.
- Adam, Z.; Aviv-Sharon, E.; Keren-Paz, A.; Naveh, L.; Rozenberg, M.; Savidor, A.; Chen, J.P. The Chloroplast Envelope Protease FTSH11-Interaction With CPN60 and Identification of Potential Substrates. Front. Plant Sci. 2019, 10, 428.
- Chen, J.; Burke, J.J.; Velten, J.; Xin, Z. FtsH11 protease plays a critical role in Arabidopsis thermotolerance. Plant J. 2006, 48, 73–84.
- Kikuchi, S.; Asakura, Y.; Imai, M.; Nakahira, Y.; Kotani, Y.; Hashiguchi, Y.; Nakai, Y.; Takafuji, K.; Bedard, J.; Hirabayashi-Ishioka, Y.; et al. A Ycf2-FtsHi Heteromeric AAA-ATPase Complex Is Required for Chloroplast Protein Import. Plant Cell 2018, 30, 2677–2703.
- Schreier, T.B.; Clery, A.; Schlafli, M.; Galbier, F.; Stadler, M.; Demarsy, E.; Albertini, D.; Maier, B.A.; Kessler, F.; Hortensteiner, S.; et al. Plastidial NAD-Dependent Malate Dehydrogenase: A Moonlighting Protein Involved in Early Chloroplast Development through Its Interaction with an FtsH12-FtsHi Protease Complex. Plant Cell 2018, 30, 1745–1769.
- Kikuchi, S.; Bedard, J.; Hirano, M.; Hirabayashi, Y.; Oishi, M.; Imai, M.; Takase, M.; Ide, T.; Nakai, M. Uncovering the Protein Translocon at the Chloroplast Inner Envelope Membrane. Science 2013, 339, 571–574.
- De Longevialle, A.F.; Hendrickson, L.; Taylor, N.L.; Delannoy, E.; Lurin, C.; Badger, M.; Millar, A.H.; Small, I. The pentatricopeptide repeat gene OTP51 with two LAGLIDADG motifs is required for the cis-splicing of plastid ycf3 intron 2 in Arabidopsis thaliana. Plant J. 2008, 56, 157–168.
- Nakai, M. New Perspectives on Chloroplast Protein Import. Plant Cell Physiol. 2018, 59, 1111–1119.
- Herrmann, J.M. A Force-Generating Machine in the Plant’s Powerhouse: A Pulling AAA-ATPase Motor Drives Protein Translocation into Chloroplasts. Plant Cell 2018, 30, 2646–2647.
- Schafer, P.; Helm, S.; Kohler, D.; Agne, B.; Baginsky, S. Consequences of impaired 1-MDa TIC complex assembly for the abundance and composition of chloroplast high-molecular mass protein complexes. PLoS ONE 2019, 14, e0213364.
- Mielke, K.; Wagner, R.; Mishra, L.S.; Demir, F.; Perrar, A.; Huesgen, P.F.; Funk, C. Abundance of metalloprotease FtsH12 modulates chloroplast development in Arabidopsis thaliana. J. Exp. Bot. 2020, 72, 3455–3473.
- Nakai, M. Reply: The Revised Model for Chloroplast Protein Import. Plant Cell 2020, 32, 543–546.
- Li, H.M.; Schnell, D.; Theg, S.M. Protein Import Motors in Chloroplasts: On the Role of Chaperones. Plant Cell 2020, 32, 536–542.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
936
Revisions:
2 times
(View History)
Update Date:
15 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




