As a highly influential physiological factor, pH may be leveraged as a tool to diagnose physiological state. It may be especially suitable for diagnosing and assessing skin structure and wound status. Multiple innovative and elegant smart wound dressings combined with either pH sensors or drug control-released carriers have been extensively studied. Increasing our understanding of the role of pH value in clinically relevant diagnostics should assist clinicians and improve personal health management in the home. In this review, we summarized a number of articles and discussed the role of pH on the skin surface as well as the factors that influence skin pH and pH-relevant skin diseases, but also the relationship of skin pH to the wound healing process, including its influence on the activity of proteases, bacterial enterotoxin, and some antibacterial agents.
1. Introduction
Skin, the major portion of the integumentary system, is the human body’s largest organ. It spans approximately 2 m
2 and is 0.075–0.15 mm thick in the average adult
[1]. It acts as the first line of defense to shield the human body from ultraviolet radiation, infection by pathogens, and chemical irritants
[2]. Furthermore, skin plays a key role in thermoregulation, immunological function, and maintaining body water balance.
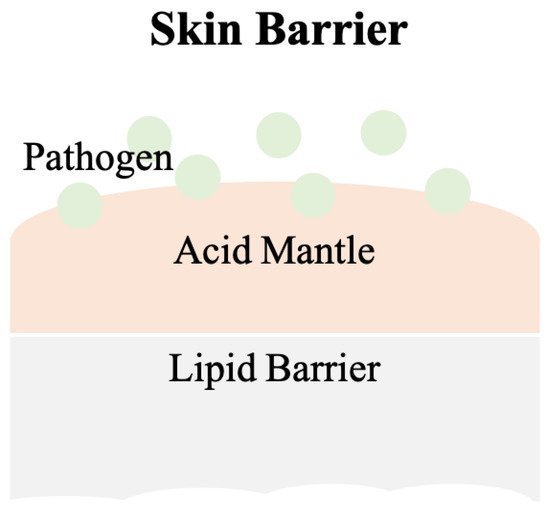
Schade and Marchionini first introduced the term “acid mantle” to describe the slight acidification of the uppermost layer of human skin
[3]. The acid mantle is characterized by a pH value of 4–6, as a result of amino acids, fatty acids, sebum secreted by the sebaceous gland, and lactate excreted from sweat. All of these compounds are acidic and when present together on the human skin, become a barrier that prevents bacterial colonization. Another critical skin barrier is the lipid barrier, the extracellular lipid matrix of the stratum corneum (SC)
[4], which is composed of free fatty acids, cholesterol and ceramides and functions as a hydrophobic barrier for human skin
[5]. A skin barrier schematic is provided in
Figure 1.
Figure 1. The scheme of the skin barrier. Lipid barrier sits atop the acid mantle and displays a pH value of 4–6. Pathogens can be various bacteria. Slight acidic environment is a disadvantage for bacterial colonization.
Most human-pathogen bacteria are inhibited by the acidic milieu on the surface of normal human skin, but this shield is disturbed when skin is wounded. The tissue beneath skin has a physiological pH of 7.4
[6], which raises the overall pH value at the wound site and provides advantages for bacterial colonization. Bacterial contaminants in wounds, e.g.,
Staphylococcus aureus, interfere with the normal wound healing process.
There are four phases in normal wound healing
[7][8]: hemostasis, inflammation, proliferation, and remodeling. During hemostasis the wound is filled with fibrin and coagulated blood. Clots are formed to stop bleeding and seal the wound site until tissues are repaired. During inflammation, histamine is produced by basophils and mast cells to increase capillary permeability, which allows leukocytes such as neutrophils to migrate to the infected wound site and remove dead cells and pathogens. However, a failure in eliminating pathogens or the remaining high pH environment can be primary reasons for chronic wound development. During proliferation, epithelial cells increase in number, granulation tissue forms, and angiogenesis occurs. Healthy granulation tissues should be pink in color and uneven in texture. The final phase, remodeling, may take years to complete. The wound matrix undergoes degradation by metalloproteinases and new extracellular matrix (ECM) is created during remodeling. Scar tissue can form due to degradation or ECM generation disruption. While the alkaline milieu activates protease, which facilitates the removal of damaged components, excessive amounts of protease eventually destroy newly constructed tissue
[9]. Moreover, alkaline pH values are usually found in chronic wounds, and are associated with an increased risk of bacterial colonization
[10].
pH value is a critical factor in the wound healing process and could offer important physiological condition information regarding skin status and infection. It assists physicians with clinically relevant diagnosis and patient wounds care. It can be used, for instance, to determine whether wounds are infected, becoming chronic, provide monitored, real-time wound status, and ultimately facilitate proper treatment in a more timely manner.
2. Skin
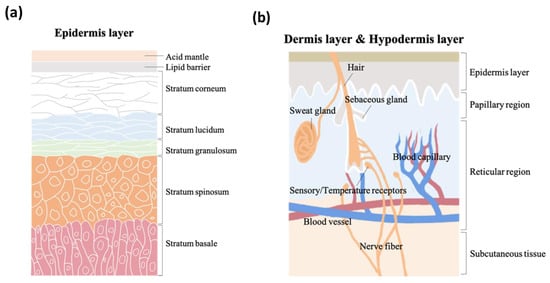
As the vital, first line of defense between the body and the environment, skin is indispensable to human life. Skin structure has been thoroughly described in the literature. It is made up of three major strata, the epidermis, the dermis, and subcutaneous tissue. The epidermis, the outermost layer in skin structure, is approximately 0.05–1 mm thick, provides barrier functions, prevents infection, and regulates transepidermal water loss (TEWL). This layer is constructed of multiple sub-layers; stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale. The acid mantle and lipid barrier can be found on top of the epidermis, specifically just above the stratum corneum.
Figure 2a is a schematic illustrating the structure of the epidermis. The second of the skin’s major strata, the dermis, can be divided into two layers, the papillary region and the reticular region. The papillary region is the thin and superficial area adjacent to the epidermis. The subsequent layer, the reticular region, is thick and contains arector pili muscles, sebaceous glands, sweat gland ducts, merocrine sweat glands, hair follicles, blood capillaries, sensory receptors, and nerve fibers. The dermis layer is approximately 1–2 mm thick, and comprises collagen, elastic fibers, and extrafibrillar matrix for providing support. The third and deepest of skin’s major three strata, subcutaneous tissue, contains fibroblasts, adipose cells, and macrophages. Subcutaneous tissue is also known as hypodermis and ranges in thickness from 1.65 mm to 18.20 mm depending on gender and skin site
[11]. The hypodermis layer is used for fat storage, but it also contains large quantities of loose connective tissue and blood vessels. The structure of the dermis and hypodermis layers are depicted in
Figure 2b.
Figure 2. The schematic structure of (a) epidermis layer, (b) dermis layer, and hypodermis layer.
2.1. pH Values on Skin Surfaces
The skin pH of newborns is weakly alkaline (pH of 7.4) as a result of being encapsulated within the amniotic fluid and the vernix caseosa. Hans, et al., determined and described skin pH characteristics from 209 newborn infants
[12]. However, after birth, the weakly alkaline skin becomes acidic in its quest to form the acid mantle
[13][14] and the functional maturation of stratum corneum is accelerated within 2 to 8 weeks
[15]. During this period, infant skin remains highly prone to damage.
During sexual maturation, the pH of skin in the axillary vault climbs from around 5.0 to near 7.0, and then returns to a lower pH during sexual involution
[16][17]. A. Zlotogorski
[18] measured the pH distribution on the surface of the skin (forehead and cheek) of 574 men and women aged 18 to 95, and found that those over the age of 80 had higher skin surface pH values compared to the other groups, which displayed skin surface pH values between 4.0 and 5.5 on the forehead and between 4.2 and 5.9 on the cheek. There was no significant pH difference based on gender. Irvin H. Blank
[19] obtained skin surface pH values (forearm, antecubital, elbow, upper arm, forehead, and back of neck) from 100 males and 100 females aged 19–27 and found pH values varying from 4.0 to 7.0, with the most frequent readings being between 4.2 and 5.6, and evidence suggesting that females have a slightly higher skin pH (0.5 higher) than males. C. Ehlers and coworkers
[20] found that there was a statistically significant difference in skin pH (arm) between men and woman. Notably, measurements could not be conducted close to the wrist because most people wash their hands several times each day, and soaps can make the skin more alkaline. Another study conducted by S. Luebberding and coworkers
[21] followed strict criteria and found pH differences between males and females in TEWL, SC hydration, sebum content, and at the skin surface (forehead, forearm, hand and cheek).
Table 1 (adapted from
[21]) displays gender-related data showing that the pH values from five skin sites and across all age groups were higher in females compared to males. This data also shows that the mean evaporimetry and corneometry values were higher for the females of this group, while the mean sebumetry values were higher for the males.
Table 1. Mean values (MV ± SD) arranged by age groups and localization (adapted from
[21]).
| |
AG I |
AG II |
AG III |
AG IV |
AG V |
Mean |
| |
♀ |
♂ |
♀ |
♂ |
♀ |
♂ |
♀ |
♂ |
♀ |
♂ |
♀ |
♂ |
| F |
5.01 ± 0.42 |
4.31 ± 0.28 |
4.98 ± 0.50 |
4.47 ± 0.33 |
4.92 ± 0.40 |
4.50 ± 0.36 |
5.10 ± 0.49 |
4.56 ± 0.30 |
4.67 ± 0.32 |
4.57 ± 0.35 |
4.94 ± 0.45 |
4.48 ± 0.33 |
| C |
5.33 ± 0.32 |
4.66 ± 0.38 |
5.27 ± 0.44 |
4.77 ± 0.33 |
5.19 ± 0.29 |
4.74 ± 0.36 |
5.35 ± 0.46 |
4.82 ± 0.27 |
5.21 ± 0.41 |
4.93 ± 0.32 |
5.27 ± 0.39 |
4.78 ± 0.34 |
| N |
5.09 ± 0.32 |
4.62 ± 0.37 |
5.09 ± 0.36 |
4.72 ± 0.36 |
5.08 ± 0.34 |
4.63 ± 0.34 |
5.37 ± 0.49 |
4.76 ± 0.28 |
5.05 ± 0.41 |
4.73 ± 0.29 |
5.13 ± 0.40 |
4.69 ± 0.33 |
| A |
5.12 ± 0.31 |
4.49 ± 0.42 |
5.22 ± 0.40 |
4.50 ± 0.36 |
5.17 ± 0.38 |
4.53 ± 0.30 |
5.44 ± 0.45 |
4.62 ± 0.37 |
5.12 ± 0.43 |
4.58 ± 0.37 |
5.21 ± 0.41 |
4.54 ± 0.36 |
| H |
5.10 ± 0.37 |
4.33 ± 0.36 |
5.19 ± 0.40 |
4.41 ± 0.32 |
5.03 ± 0.35 |
4.38 ± 0.31 |
5.25 ± 0.38 |
4.55 ± 0.42 |
4.88 ± 0.41 |
4.46 ± 0.40 |
5.09 ± 0.40 |
4.42 ± 0.37 |
| |
Female (Age ± SD) |
Male (Age ± SD) |
| AG I |
24.73 ± 2.59 |
25.70 ± 2.48 |
| AG II |
33.30 ± 2.92 |
33.23 ± 2.90 |
| AG III |
43.63 ± 2.81 |
44.23 ± 3.09 |
| AG IV |
55.17 ± 2.84 |
54.20 ± 3.13 |
| AG V |
66.57 ± 4.31 |
66.63 ± 2.61 |
It is common to obtain inconsistent pH measurements from men and women, but additional research could provide data to support a pattern of pH value differences between genders.
2.2. Factors That Affects Skin pH
Regulating skin surface pH is critical to physiological defense mechanisms, but skin is affected by a number of endogenous and exogenous factors
[22], including age, ethnicity, sebum, sweat, detergents, cosmetic products, soaps, occlusive dressing, and more. These factors and their influence are displayed in
Table 2 [18][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42].
Table 2. Factors that have influences on skin pH.
| Factors |
Influences |
References |
| Age |
Increased age is associated with skin pH values that are in steady fluctuation. This contributes to difficulties in making comparisons. Females in [21] show significantly higher skin pH values than males. |
[18][21][36] |
| Ethnic |
Whites have slightly higher pH than blacks, but there are mostly no statistically significant differences between ethnic groups. |
[37][38][39] |
| Sebum |
There is a negative correlation between sebum secretion and skin pH that is statistically significant in only the skin U-zone with acne and in the skin T-zone without acne, yet the correlation is not strong enough to explain the impact of sebum secretion on skin pH. |
[40] |
| Sweat |
Sweat is predominantly composed of sodium chloride, lactic acid, urea, and fatty acid. The acidic nature of human skin is partially due to lactic acid in sweat. As the rate of eccrine sweat increases, the concentrations of ammonia, pyruvic acid and lactate decrease, hence the pH increases. |
[23][24][25][41][42] |
| Soaps and detergents |
The use of soaps, synthetic detergents and even tap water will raise skin pH in a short-term effect. |
[22][26][27][28][29][30] |
| Cosmetic products |
Skin pH values drop after the application of cosmetic products, which can buffer the impact of tap water washing. |
[31][32] |
| Occlusive dressings |
Under occlusion, skin pH increases, but after removing occlusive dressings, the pH milieu drops back to an acidic nature. The application of occlusion has a short period effect on skin pH values. |
[33][34][35] |
Elizabeth A. and coworkers
[43] analyzed the distribution of the human skin microbiome via examination of 16S ribosomal RNA gene sequences, and found that bacteria in sebaceous sites where
Propionibacterium acnes predominated were less diverse, less even, and less rich than the bacterial milieu in moist and dry sites, which were predominantly associated with Firmicutes and Proteobacteria. Multiple factors such as sweat, sebum secretion, and pH could have correlation with predominant microbiota. This study revealed a pattern of bacterial distribution on human skin that may have implications for the future treatment of pH-relevant skin disorders, a topic that we will explore further.
2.3. Skin Diseases and pH Values
Beneath the acid mantle and lipid barrier, the various strata of skin have relatively static and regulated physiological pH values, but when disruptions in the protective barriers occur, or when normal skin pH is disrupted, the risk for specific skin diseases increases. Many studies have suggested a relationship between skin surface pH and disease.
It has been reported that great numbers of
Staphylococcus aureus exist on the skin of patients with eczema can exacerbate the disease
[44].
Staphylococcus aureus has a capacity for adhering to atopic corneocytes; this adherence phenomenon is partially attributable to the presence of protein A in its wall
[45]. The pH range for optimal growth of
Staphylococcus aureus is between 7.0 and 7.5, yet the bacteria are able to reproduce at pH values from 4.5 to 9.0. The pH value of the normal skin surface, 4.5–6.0, is consequently insufficient to kill the bacteria completely. However, the staphylococcal enterotoxin C2 (SEC2) can be destroyed in strongly acidic pH. Zinc ion, present in the crystalline structure of SEC2, may be removed completely in this process, which distorts SEC2 from its normal configuration
[46].
pH on the skin surface increases in patients with seborrheic dermatitis
[47][48][49].
Pityrosporum ovule prosper in scale of patients with dandruff, since higher pH values also promote the growth of yeast. Its colonization in less acidic areas of the skin result in declining activity of calf thymus histones, parotid saliva, lysozyme, and proteins obtained from human neutrophil granulocyte, which are cationic substances critically related to bacterial activity
[50][51][52][53].
Diaper dermatitis is frequently diagnosed in infants, and wearing diapers is associated with increases in wetness and skin pH value. Ronald and coworkers
[54] carried out a study involving 1601 infants. Their results suggest that diapered skin has approximately twice the TEWL of undiapered skin. Diapered skin has a higher pH value (5.9) while undiapered skin (pH 5.3) remains related to the acid mantle pH. Skin wetness could disturb the integrity of skin by increasing its permeability to irritants, frictional coefficient, and promoting microbial growth
[55]. Elevated pH can increase the activity of fecal proteolytic enzymes that can degrade the extracellular matrix of the skin and eventually destroy tissues
[56].
Skin pH is believed to be one of possible factors that promote candida infection, which diabetics are particularly prone to suffer from. Dimorphism of
Candida albicans exists, as the blastospore form is related to acidic pH and the mycelial form is associated with alkaline pH
[57][58]. The mycelial form of
Candida albicans has been verified in clinical studies via its pathogenicity
[59][60][61]. Gil Yosopovitch and coworkers
[62] found that diabetic patients had significantly higher skin pH than that of normal control skin. Moreover, the skin pH values of diabetic patients with candidiasis was higher than that of diabetic patients without candidiasis. This suggests a possible correlation between skin pH and candida infection.
2.4. Skin Care
There are several structural differences between the skin of newborns and that of adults. Because newborn skin has a poorly functional skin barrier, and because newborns have fragile skin that can be easily damaged, proper and adequate steps must be taken to provide protection. S. Dhar
[63] suggested that newborn bathing should not last longer than 5 min, or it could increase the hydration of the skin and decrease the friction threshold. Moreover, soaps and cleansers should be minimally used in the first few weeks of life for newborns, because any attempt intended to raise the skin pH would promote the number of bacteria and increase the TEWL
[64]. There is not enough evidence that soaps have long-term impact on infants, but in the short term, it could disturb their acid mantles
[26][28][29]. Synthetic detergents such as cocoyl isethionate, and sodium lauryl sulphate are soap substitutes that have pH values similar to skin and are more moderate than soaps. They do not alter skin pH and microflora, but they disintegrate rapidly and can cause skin dryness
[65].
The spread of lesions in infantile seborrheic dermatitis can be limited by mineral oil or vegetable oil and minimizing the use of shampoos for cleaning the hair. Sarkar et al.
[66] recommended the use of coconut oil as an emollient for application to neonatal skin due to its easy availability and economy. Skin irritation can also be avoided by using fragrance-free baby products.
For adult skin care, Y.C. Jung and coworkers
[67] suggested that low skin pH could be maintained by increasing hydration, the presence of low skin pH induced higher sebum excretion rate, and the combined effects could suppress skin aging by reducing wrinkle length and depth. In work by S. H. Youn et al.
[68], they found it helpful to control acne in the T-zone by increasing skin pH to 5.5–6 for female acne patients, and by decreasing skin pH to approximately 5.5 for male patients. Saba et al.
[69] recommended that the ideal pH for body wash, soap, or cleanser would be in the range of 4.5–6.5, which is similar to that of the skin’s acid mantle. Moreover, syndets, synthetic detergents, are less irritating and preferred. Nix and coworkers
[70] suggested that the selection of skin cleaning products should take multiple factors into consideration, namely formulation, ingredients, skin compatibility, pH, and related infection control issues. Skin cleaning products with a pH value higher than 7 can disrupt skin barrier function. It is particularly important for elderly patients to select products with a pH value of 4–7 because the skin of the elderly is dryer, more prone to cracking, and recovers more slowly from damage caused by alkalinity.
3. Wounds
Wounds are divided into two categories, acute and chronic. Acute wounds can be healed predictably with normal wound healing approaches, and chronic wounds develop following one or more failures in the normal wound healing process. The wound healing process has four phases and has been described in detail above.
Acute wounds, sudden injuries to the skin, vary from superficial scratches to deep skin damages that can happen anywhere on the body. The causes of acute wounds vary but primarily include abrasion, puncture, laceration, and incision. Classified by causality, the two dominant types of acute wounds are surgical and traumatic. Surgical wounds are intentionally created for medical reasons, and traumatic wounds are randomly caused by external force. Severe pain is associated with wounds, which are especially prevalent among patients with fragile skin.
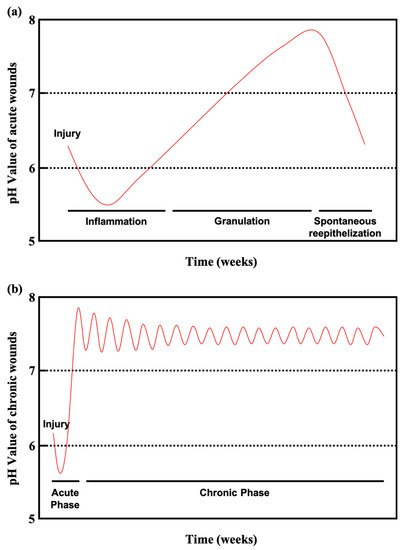
Chronic wounds remain in an inflamed state that delays healing for long periods lasting several months. Chronic wounds may take years to recover or may never heal, leading to physical and psychological suffering as well as considerable pressure on the social healthcare system. Diabetic patients with chronic wounds are at especially high risk for bacterial infections, because the slightly alkaline pH of chronic wounds promotes bacterial colonization.
Figure 3a shows the pH values of acute wounds and chronic wounds in
Figure 3b (adapted from
[10]). During healing, the pH of acute wounds gradually declines to a level approximating that of the acid mantle. Chronic wounds, however, remain slightly alkaline. Monitoring and manipulating pH can be a critical tool for preventing and treating chronic wounds. Studies have reported on pH value monitoring combined with pH-activated drug control release systems and smart wound dressings and bandages
[71][72][73][74][75].
Figure 3. (
a) Course of the pH milieu in acute wounds. (
b) Course of the pH milieu in chronic wounds (adapted from
[10]).
Severe chronic wounds with bacterial infections can evolve into bacteremia, sepsis, or septicemia and severe deterioration in wound status. Severely chronic wounds can result in amputation, a frequent occurrence with diabetic foot ulcers, and a major issue in modern healthcare
[76][77]. Diabetic patient wound care management must be undertaken with care and rigor. In 2014, chronic wounds impacted 15% of all Medicare beneficiaries in the United States, with an estimated cost of $28–$32 billion annually
[78]. Among the 15% of Medicare beneficiaries (8.2 million in population), who had at least one type of wound or infection, surgical infections were most prevalent (4.0%), followed by diabetic infections (3.4%)
[79]. The huge cost of nonhealing chronic wounds has become an urgent issue that should be taken seriously.