
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea Piccioni | + 2902 word(s) | 2902 | 2021-08-03 04:25:00 | | | |
| 2 | Bruce Ren | -21 word(s) | 2881 | 2021-08-12 11:03:44 | | |
Video Upload Options
The diagnosis and treatment of sepsis have always been a challenge for the physician, especially in critical care setting such as emergency department (ED), and currently sepsis remains one of the major causes of mortality. Although the traditional definition of sepsis based on systemic inflammatory response syndrome (SIRS) criteria changed in 2016, replaced by the new criteria of SEPSIS-3 based on organ failure evaluation, early identification and consequent early appropriated therapy remain the primary goal of sepsis treatment. Unfortunately, currently there is a lack of a foolproof system for making early sepsis diagnosis because conventional diagnostic tools like cultures take a long time and are often burdened with false negatives, while molecular techniques require specific equipment and have high costs. In this context, biomarkers, such as C-Reactive Protein (CRP) and Procalcitonin (PCT), are very useful tools to distinguish between normal and pathological conditions, graduate the disease severity, guide treatment, monitor therapeutic responses and predict prognosis.
1. Introduction
2. Current Data on Presepsin as a Biomarker for Sepsis
2.1. Presepsin
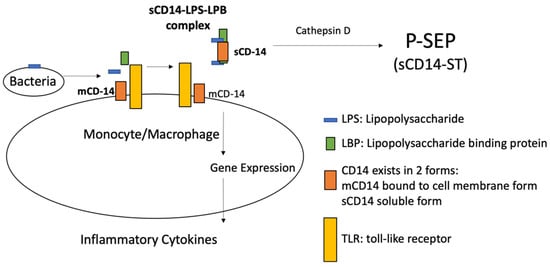
2.2. Presepsin Measurement
2.3. Diagnostic Significance of Presepsin in Sepsis
2.4. Prognostic Significance of Presepsin in Sepsis
2.5. Presepsin Compared to C-RP and PCT: Alone or in Company?
2.6. Presepsin in Pediatric Bacterial Infection
2.7. Presepsin in Fungal Infection
2.8. Presepsin Significance in SARS-CoV-2 Infection
2.9. Presepsin in Emergency Department
2.10. Presepsin Use Caveat
References
- Ulla, M.; Pizzolato, E.; Lucchiari, M.; Loiacono, M.; Soardo, F.; Forno, D.; Morello, F.; Lupia, E.; Moiraghi, C.; Mengozzi, G.; et al. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: A multicenter prospective study. Crit. Care 2013, 17, R168.
- Balci, C.; Sungurtekin, H.; Gürses, E.; Sungurtekin, U.; Kaptanoğlu, B. Usefulness of procalcitonin for diagnosis of sepsis in the intensive care unit. Crit. Care 2002, 7, 85–90.
- Harbarth, S.; Holeckova, K.; Froidevaux, C.; Pittet, D.; Ricou, B.; Grau, G.E.; Vadas, L.; Pugin, J.; Network, G.S. Diagnostic value of procalcitonin, interleukin-6, and in- terleukin-8 in critically ill patients admitted with suspected sepsis. Am. J. Respir. Crit. Care Med. 2001, 164, 396–402.
- Yusa, T.; Tateda, K.; Ohara, A.; Miyazaki, S. New possible biomarkers for diagnosis of infections and diagnostic distinction between bacterial and viral infections in children. J. Infect. Chemother. 2017, 23, 96–100.
- Alizadeh, N.; Memar, M.Y.; Moaddab, S.R.; Kafil, H.S. Aptamer-assisted novel technologies for detecting bacterial pathogens. Biomed. Pharmacother. 2017, 93, 737–745.
- Leli, C.; Ferranti, M.; Marrano, U.; Al Dhahab, Z.S.; Bozza, S.; Cenci, E.; Mencacci, A. Diagnostic accuracy of presepsin (sCD14-ST) and procalcitonin for prediction of bacteraemia and bacterial DNAaemia in patients with suspected sepsis. J. Med. Microbiol. 2016, 65, 713–719.
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992, 101, 1644–1655.
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810.
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87.
- Labib, M.; Berezovski, M.V. Electrochemical aptasensors for microbial and viral pathogens. Adv. Biochem. Eng. Biotechnol. 2014, 140, 155–181.
- Biron, B.M.; Ayala, A.; Lomas-Neira, J.L. Biomarker insight biomarkers for sepsis: What is and what might be? Biomark Insights 2015, 10 (Suppl. 4), 7–17.
- Pierrakos, C.; Vincent, J.-L. Sepsis biomarkers: A review. Crit. Care 2010, 14, R15.
- Chenevier-Gobeaux, C.; Borderie, D.; Weiss, N.; Mallet-Coste, T.; Claessens, Y.-E. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin. Chim. Acta 2015, 450, 97–103.
- Memar, M.Y.; Baghi, H.B. Presepsin: A promising biomarker for the detection of bacterial infections. Biomed. Pharmacother. 2019, 111, 649–656.
- Zhang, J.; Hu, Z.-D.; Song, J.; Shao, J. Diagnostic Value of Presepsin for Sepsis. A Systematic Review and Meta-Analysis. Medicine 2015, 94, e2158.
- Memar, M.Y.; Alizadeh, N.; Varshochi, M.; Kafil, H.S. Immunologic biomarkers for diagnostic of early-onset neonatal sepsis. J. Matern. Neonatal Med. 2017, 32, 143–153.
- Sandquist, M.; Wong, H.R. Biomarkers of sepsis and their potential value in diagnosis, prognosis and treatment. Expert Rev. Clin. Immunol. 2014, 10, 1349–1356.
- Vanaja, S.K.; Rathinam, V.A.; Fitzgerald, K.A. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol. 2015, 25, 308–315.
- Lonez, C.; Irvine, K.; Pizzuto, M.; Schmidt, B.I.; Gay, N.J.; Ruysschaert, J.-M.; Gangloff, M.; Bryant, C.E. Critical residues involved in Toll-like receptor 4 activation by cationic lipid nanocarriers are not located at the lipopolysaccharide-binding interface. Cell. Mol. Life Sci. 2015, 72, 3971–3982.
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353.
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801.
- Nonaka, M.; Yoshizaki, F. Evolution of the complement system. Mol. Immunol. 2004, 40, 897–902.
- Barton, G.M.; Medzhitov, R. Control of adaptive immune responses by Toll-like receptors. Curr. Opin. Immunol. 2002, 14, 380–383.
- Van Der Mark, V.A.; Ghiboub, M.; Marsman, C.; Zhao, J.; Van Dijk, R.; Hiralall, J.K.; Ho-Mok, K.S.; Castricum, Z.; De Jonge, W.J.; Elferink, R.P.J.O.; et al. Phospholipid flippases attenuate LPS-induced TLR4 signaling by mediating endocytic retrieval of Toll-like receptor 4. Cell. Mol. Life Sci. 2017, 74, 715–730.
- Yuk, J.-M.; Jo, E.-K. Toll-like Receptors and Innate Immunity. J. Bacteriol. Virol. 2011, 41, 225–235.
- Zou, Q.; Wen, W.; Zhang, X.-C. Presepsin as a novel sepsis biomarker. World J. Emerg. Med. 2014, 5, 16–19.
- Shirakawa, K.; Naitou, K.; Hirose, J.; Takahashi, T.; Furusako, S. Prese-psin [sCD14-ST]: Development and evaluation of one-step ELISA with a new standard that is similar to the form of presepsin in septic patients. Clin. Chem. Lab. Med. 2011, 49, 937–939.
- Okamura, Y.; Yokoi, H. Development of a point-of-care assay system for measurement of presepsin (sCD14-ST). Clin. Chim. Acta 2011, 412, 2157–2161.
- Novelli, G.; Morabito, V.; Ferretti, G.; Pugliese, F.; Ruberto, F.; Venuta, F.; Poli, L.; Rossi, M.; Berloco, P. Pathfast Presepsin Assay for Early Diagnosis of Bacterial Infections in Surgical Patients: Preliminary Study. Transplant. Proc. 2013, 45, 2750–2753.
- Pugni, L.; Pietrasanta, C.; Milani, S.; Vener, C.; Ronchi, A.; Falbo, M.; Arghittu, M.; Mosca, F. Presepsin (Soluble CD14 Subtype): Reference Ranges of a New Sepsis Marker in Term and Preterm Neonates. PLoS ONE 2015, 10, e0146020.
- Endo, S.; Suzuki, Y.; Takahashi, G.; Shozushima, T.; Ishikura, H.; Murai, A.; Nishida, T.; Irie, Y.; Miura, M.; Iguchi, H.; et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J. Infect. Chemother. 2012, 18, 891–897.
- Liu, B.; Chen, Y.-X.; Yin, Q.; Zhao, Y.-Z.; Li, C.-S. Diagnostic value and prognostic evaluation of Presepsin for sepsis in an emergency department. Crit. Care 2013, 17, R244.
- Topcuoğlu, S.; Arslanbuga, C.; Gursoy, T.; Aktas, A.; Karatekin, G.; Uluhan, R.; Ovali, F. Role of presepsin in the diagnosis of late-onset neonatal sepsis in preterm infants. J. Matern. Neonatal Med. 2015, 29, 1–6.
- Vodnik, T.; Kaljevic, G.; Tadic, T.; Majkic-Singh, N. Presepsin (sCD14-ST) in pre-operative diagnosis of abdominal sepsis. Clin. Chem. Lab. Med. 2013, 51, 2053–2062.
- Masson, S.; Caironi, P.; Fanizza, C.; Thomae, R.; Bernasconi, R.; Noto, A.; Oggioni, R.; Pasetti, G.S.; Romero, M.; Tognoni, G.; et al. Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: Data from the multicenter, randomized ALBIOS trial. Intensiv. Care Med. 2015, 41, 12–20.
- Wu, J.; Hu, L.; Zhang, G.; Wu, F.; He, T. Accuracy of Presepsin in Sepsis Diagnosis: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0133057.
- Popa, T.O.; Cimpoeşu, D. Dorobăţ CM: Diagnostic and prognostic value of presepsin in the emergency department. Rev Med. Chir. Soc. Med. Nat. Iasi. 2015, 119, 69–76.
- Carpio, R.; Zapata, J.; Spanuth, E.; Hess, G. Utility of presepsin (sCD14-ST) as a diagnostic and prognostic marker of sepsis in the emergency department. Clin. Chim. Acta 2015, 450, 169–175.
- Velissaris, D.; Zareifopoulos, N.; Karamouzos, V.; Karanikolas, E.; Pierrakos, C.; Koniari, I.; Karanikolas, M. Presepsin as a Diagnostic and Prognostic Biomarker in Sepsis. Cureus 2021, 13, 15019.
- Yang, H.S.; Hur, M.; Yi, A.; Kim, H.; Lee, S.; Kim, S.-N. Prognostic value of presepsin in adult patients with sepsis: Systematic review and meta-analysis. PLoS ONE 2018, 13, e0191486.
- Kweon, O.J.; Choi, J.-H.; Park, S.K.; Park, A.J. Usefulness of presepsin (sCD14 sub- type) measurements as a new marker for the diagnosis and prediction of disease severity of sepsis in the Korean population. J. Crit. Care 2014, 29, 965–970.
- Memar, M.Y.; Ghotaslou, R.; Samiei, M.; Adibkia, K. Antimicrobial use of reactive oxygen therapy: Current insights. Infect. Drug Resist. 2018, 11, 567–576.
- Godnic, M.; Stubljar, D.; Skvarc, M.; Jukic, T. Diagnostic and prognostic value of sCD14-ST—presepsin for patients admitted to hospital intensive care unit (ICU). Wien. Klin. Wochenschr. 2015, 127, 521–527.
- Hung, S.-K.; Lan, H.-M.; Han, S.-T.; Wu, C.-C.; Chen, K.-F. Current Evidence and Limitation of Biomarkers for Detecting Sepsis and Systemic Infection. Biomedicines 2020, 8, 494.
- Zhu, Y.; Li, X.; Guo, P.; Chen, Y.; Li, J.; Tao, T. The accuracy assessment of presepsin (sCD14-ST) for mortality prediction in adult patients with sepsis and a head-to-head comparison to PCT: A meta-analysis. Ther. Clin. Risk Manag. 2019, 15, 741–753.
- Kim, H.; Hur, M.; Moon, H.-W.; Yun, Y.-M.; Di Somma, S.; Network, G. Multi-marker approach using procalcitonin, presepsin, galectin-3, and soluble suppression of tumorigenicity 2 for the prediction of mortality in sepsis. Ann. Intensiv. Care 2017, 7, 27.
- Madenci, Ö.Ç.; Yakupoğlu, S.; Benzonana, N.; Yücel, N.; Akbaba, D.; Kaptanağası, A.O. Evaluation of soluble CD14 subtype (presepsin) in burn sepsis. Burns 2014, 40, 664–669.
- De Guadiana Romualdo, L.G.; Torrella, P.E.; González, M.V.; Sánchez, R.J.; Holgado, A.H.; Freire, A.O.; Acebes, S.R.; Otón, M.D.A. Diagnostic accuracy of pre-sepsin (soluble CD14 subtype) for prediction of bacteremia in patients with systemic inflammatory response syndrome in the Emergency Department. Clin. Biochem. 2014, 47, 505–508.
- Romualdo, L.G.D.G.; Torrella, P.E.; Acebes, S.R.; Otón, M.D.A.; Sánchez, R.J.; Holgado, A.H.; Santos, E.J.; Freire, A.O. Diagnostic accuracy of presepsin (sCD14-ST) as a biomarker of infection and sepsis in the emergency department. Clin. Chim. Acta 2017, 464, 6–11.
- Enguix-Armada, A.; Escobar-Conesa, R.; García-De La Torre, A.; De La Torre- Prados, M.V. Usefulness of several biomarkers in the management of septic patients: C- reactive protein, procalcitonin, presepsin and mid-regional pro-adrenomedullin. Clin. Chem. Lab. Med. (CCLM) 2016, 54, 163–168.
- Plesko, M.; Suvada, J.; Makohusova, M.; Waczulikova, I.; Behulova, D.; Vasilenkova, A.; Vargova, M.; Stecova, A.; Kaiserova, E.; Kolenova, A. The role of CRP, PCT, IL-6 and presepsin in early diagnosis of bacterial infectious complications in paediatric haemato-oncological patients. Neoplasma 2016, 63, 752–760.
- Klouche, K.; Cristol, J.P.; Devin, J.; Gilles, V.; Kuster, N.; Larcher, R.; Amigues, L.; Corne, P.; Jonquet, O.; Dupuy, A.M. Diagnostic and prognostic value of soluble CD14 subtype (Presepsin) for sepsis and community-acquired pneumonia in ICU patients. Ann. Intensiv. Care 2016, 6, 1–11.
- Parri, N.; Trippella, G.; Lisi, C.; De Martino, M.; Galli, L.; Chiappini, E. Accuracy of presepsin in neonatal sepsis: Systematic review and meta-analysis. Expert Rev. Anti. Infect Ther. 2019, 17, 223–232.
- Van Maldeghem, I.; Nusman, C.M.; Visser, D.H. Soluble CD14 subtype (sCD14-ST) as biomarker in neonatal early-onset sepsis and late-onset sepsis: A systematic review and meta-analysis. BMC Immunol. 2019, 20, 17.
- Iskandar, A.; Arthamin, M.Z.; Indriana, K.; Anshory, M.; Hur, M.; Di Somma, S.; Network, O.B.O.T.G. Comparison between presepsin and procalcitonin in early diagnosis of neonatal sepsis. J. Matern. Neonatal Med. 2018, 32, 3903–3908.
- Kumar, N.; Dayal, R.; Singh, P.; Pathak, S.; Pooniya, V.; Goyal, A.; Kamal, R.; Mohanty, K.K. A Comparative Evaluation of Presepsin with Procalcitonin and CRP in Diagnosing Neonatal Sepsis. Indian J. Pediatr. 2019, 86, 177–179.
- Baraka, A.; Zakaria, M. Presepsin as a diagnostic marker of bacterial infections in febrile neutropenic pediatric patients with hematological malignancies. Int. J. Hematol. 2018, 108, 184–191.
- El Gendy, F.M.; El-Mekkawy, M.S.; Saleh, N.Y.; Habib, M.S.E.-D.; Younis, F.E. Clinical study of Presepsin and Pentraxin3 in critically ill children. J. Crit. Care 2018, 47, 36–40.
- Mussap, M.; Puxeddu, E.; Puddu, M.; Ottonello, G.; Coghe, F.; Comite, P.; Cibecchini, F.; Fanos, V. Soluble CD14 subtype (sCD14-ST) presepsin in premature and full term critically ill newborns with sepsis and SIRS. Clin. Chim. Acta 2015, 451, 65–70.
- Koh, H.; Aimoto, M.; Katayama, T.; Hashiba, M.; Sato, A.; Kuno, M.; Makuuchi, Y.; Takakuwa, T.; Okamura, H.; Hirose, A. Diagnostic value of levels of presepsin (so- luble CD14-subtype) in febrile neutropenia in patients with hematological dis- orders. J. Infect. Chemother. 2016, 22, 466–471.
- Poggi, C.; Bianconi, T.; Gozzini, E.; Generoso, M.; Dani, C. Presepsin for the Detection of Late-Onset Sepsis in Preterm Newborns. Pediatrics 2015, 135, 68–75.
- Ruan, L.; Chen, G.-Y.; Liu, Z.; Zhao, Y.; Xu, G.-Y.; Li, S.-F.; Li, C.-N.; Chen, L.-S.; Tao, Z. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: A meta-analysis and systematic review. Crit. Care 2018, 22, 1–9.
- Yoon, S.H.; Kim, E.H.; Kim, H.Y.; Ahn, J.G. Presepsin as a diagnostic marker of sepsis in children and adolescents: A systemic review and meta-analysis. BMC Infect Dis. 2019, 19, 760.
- Kollmann, T.R.; Levy, O.; Montgomery, R.; Goriely, S. Innate Immune Function by Toll-like Receptors: Distinct Responses in Newborns and the Elderly. Immunity 2012, 37, 771–783.
- Badiee, P.; Hashemizadeh, Z. Opportunistic invasive fungal infections: Diagnosis & clinical management. Indian J. Med. Res. 2014, 139, 195–204.
- Stoma, I.; Karpov, I.; Uss, A.; Krivenko, S.; Iskrov, I.; Milanovich, N.; Vlasenkova, S.; Lendina, I.; Belyavskaya, K.; Cherniak, V. Combination of sepsis biomarkers may indicate an invasive fungal infection in haematological patients. Biomarkers 2019, 24, 401–406.
- Lippi, G.; Cervellin, G. Can presepsin be used for screening invasive fungal infections? Ann. Transl. Med. 2019, 7, 87.
- Bamba, Y.; Moro, H.; Aoki, N.; Koizumi, T.; Ohshima, Y.; Watanabe, S. Increased presepsin levels are associated with the severity of fungal bloodstream infections. PLoS ONE 2019, 13, e0206089.
- Fukada, A.; Kitagawa, Y.; Matsuoka, M.; Sakai, J.; Imai, K.; Tarumoto, N.; Orihara, Y.; Kawamura, R.; Takeuchi, S.; Maesaki, S.; et al. Presepsin as a predictive biomarker of severity in COVID-19: A case series. J. Med. Virol. 2021, 93, 99–101.
- Zaninotto, M.; Mion, M.M.; Cosma, C.; Rinaldi, D.; Plebani, M. Presepsin in risk stratification of SARS-CoV-2 patients. Clin. Chim. Acta 2020, 507, 161–163.
- Nagata, T.; Yasuda, Y.; Ando, M.; Abe, T.; Katsuno, T.; Kato, S.; Tsuboi, N.; Matsuo, S.; Maruyama, S. Clinical Impact of Kidney Function on Presepsin Levels. PLoS ONE 2015, 10, e0129159.
- Sargentini, V.; Ceccarelli, G.; D’Alessandro, M.; Collepardo, D.; Morelli, A.; D’Egidio, A.; Mariotti, S.; Nicoletti, A.M.; Evangelista, B.; D’Ettorre, G.; et al. Presepsin as a potential marker for bacterial infection relapse in critical care patients. A preliminary study. Clin. Chem. Lab. Med. 2014, 53, 567–573.
- Hayashi, M.; Yaguchi, Y.; Okamura, K.; Goto, E.; Onodera, Y.; Sugiura, A.; Suzuki, H.; Nakane, M.; Kawamae, K.; Suzuki, T. A case of extensive burn without sepsis showing high level of plasma presepsin (sCD14-ST). Burn. Open 2017, 1, 33–36.




