Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Natalia A. Shnayder | + 2924 word(s) | 2924 | 2021-10-09 05:05:03 | | | |
| 2 | Amina Yu | Meta information modification | 2924 | 2021-10-21 03:37:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shnayder, N. Skin Collagen Fiber Turnover and Functioning. Encyclopedia. Available online: https://encyclopedia.pub/entry/15185 (accessed on 08 February 2026).
Shnayder N. Skin Collagen Fiber Turnover and Functioning. Encyclopedia. Available at: https://encyclopedia.pub/entry/15185. Accessed February 08, 2026.
Shnayder, Natalia. "Skin Collagen Fiber Turnover and Functioning" Encyclopedia, https://encyclopedia.pub/entry/15185 (accessed February 08, 2026).
Shnayder, N. (2021, October 20). Skin Collagen Fiber Turnover and Functioning. In Encyclopedia. https://encyclopedia.pub/entry/15185
Shnayder, Natalia. "Skin Collagen Fiber Turnover and Functioning." Encyclopedia. Web. 20 October, 2021.
Copy Citation
Clinically, collagen formation disorders manifest themselves as increased flabbiness and looseness of the skin and as early signs of facial aging. In addition to the clinical picture, it is important for cosmetologists and dermatologists to understand the etiology and pathogenesis of collagenopathies. This entry summarized and systematized the available information concerning the role of genetic and epigenetic factors in skin collagen fiber turnover.
skin collagen
collagen synthesis
collagen degradation
skin collagen functions
collagenopathy
gene polymorphism
aging
personalized dermatology
personalized cosmetology
1. Introduction
To prescribe pathogenetically grounded therapy for aesthetic skin imperfections, it is important to understand the physiological and pathological processes in the skin and, on this basis, prescribe a set of measures aimed at restoring the skin’s physiological properties [1]. It is thus necessary to perform an in-depth study of collagen fiber turnover, including the genetic aspects of collagen formation. The synthesis of the existing data concerning the genes that encode key proteins and enzymes at all stages of skin collagen fiber turnover can help develop new predictive strategies in medical cosmetology (aesthetic medicine).
Collagen makes up 25% (in dry weight) of all proteins in the human body, constituting the basis of connective tissue, including skin [2]. Many modern methods of aesthetic medicine are aimed at improving or stimulating the synthesis of collagen fibers in the skin [3]. Furthermore, various companies have undertaken clinical trials and histological studies and have suggested that particular techniques bring significant results. However, in practice, we are far from achieving consistent and significant clinical effects in all patients.
In the context of obtaining diverse results in our patients, we often speak of “individual characteristics” of a particular person, but what lies at the core of such individual, personal, characteristics?
There are two groups of factors that can influence the synthesis of collagen in the skin: external and internal [4]. External factors include the type of diet (the completeness of the intake of nutrients necessary for collagen synthesis) and the impact of environmental factors. Internal factors include the state of the hormonal background, the inherent genetic sequences encoding the structural elements of the skin, and epigenetic regulation of the activity of genes encoding key proteins and enzymes of collagen formation [5].
The extracellular matrix of the skin can regulate cell morphology, proliferation, migration, and gene expression [6]. Disruption of collagen fiber turnover can lead to damage to skin architectonics, reduction regeneration, a response to aesthetic procedures, and the formation of pathological fibrosis [7]. Moreover, pathological “vicious circles” occur when a disruption in the state of the extracellular matrix leads to a decrease in the synthetic activity of fibroblasts and an even greater disruption of the extracellular matrix structure; thickening of collagen fibers (in the case of pathological fibrosis) leads to the differentiation of fibroblasts into α-smooth muscle actin-positive contractile myofibroblasts, resulting in an even greater severity of pathological fibrosis [8][9]. Thus, understanding and taking into account the genetic predictors of the metabolism of collagen fibers in the skin underlie the personalized management of patients by dermatologists and cosmetologists [10].
The genetic aspects of collagen fiber turnover (synthesis, functioning, and degradation) and their role in health and disease are under active investigation. The majority of studies are devoted to collagen in bone tissue and internal organs. The number of studies concerning genetic predictors of collagen formation in the skin has been increasing in recent years, but there is a need to systematize the existing data.
2. Collagen Fibers in the Skin
One of the most important functions of the skin, protection from mechanical damage, is realized through the reversible deformation of the structure, and this is made possible, to a large extent, due to collagen fibers [11]. There are ethnic features relating to the structure of collagen fibers and the cellular composition of the dermis, but with age, the skin becomes thinner, more rigid, less tense, and less elastic [12]. Changes also occur in the structure of collagen fibers. Specifically, with age, collagen fibers become more disorganized; in the papillary dermis, the diameter of collagen fibers decreases. In the reticular layer, the diameter of collagen fibers increases in people up to 45 years of age, and then it decreases and the collagen fibers become coarser and more rigid [13].
Collagen fibers make up most of the dermis and form a highly organized three-dimensional scaffold that surrounds cells. Between them, there are a large number of various macromolecules that bind water (glycosaminoglycans, fibronectin, tenascin, fibronectin, epimorphin, and others) [14]. Collagen fibers have a uniform orientation and provide passive tension, which causes internal skin tension along Langer’s lines [15]. Furthermore, collagen bundles are connected by elastic fibers, which perform the function of adaptation to deformation (returning collagen fibers to their original state after the termination of the load) [16].
In addition to its mechanical function, collagen plays a key role in the regulation of cell migration and differentiation, and it has a signaling function as proteins of the cell surface bind to it [17]. Collagen’s interaction with cell surface proteins can be carried out through receptors that recognize amino acid sequences on the collagen molecule. Moreover, certain proteins can bind to both collagen and integrins, promoting cell adhesion and proliferation [18][19]. When collagen fibers disintegrate, peptide regulatory factors are released that affect further regeneration [20].
In humans, like in other vertebrates, 29 types of collagens have currently been described, encoded by at least 45 different genes [21]. The composition of collagen fibers varies in different organs depending on the functions of the corresponding organ [22]. The skin, which bears mechanical stress, is dominated by fibrillar collagens (including a large amount of type I collagen and a small amount of types III and V) (Figure 1). The activity related to the synthesis of different skin collagen types can be estimated by RPKM (reads per kilobase per million mapped reads) (Table 1).
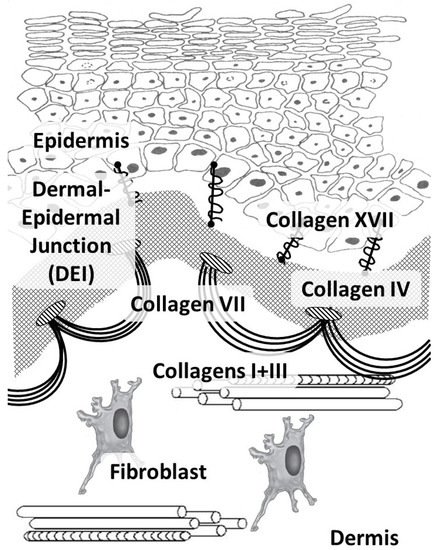
Figure 1. Main skin collagens: dermal (types I and III) and dermal-epidermal junction (types IV, VII, and XVII) collagens.
Collagens of types I, III, and V belong to the group of fibrillar collagens. Moreover, collagen type I is distributed in many tissues, such as the skin, bone tissue, the cornea and sclera of the eye, and the walls of blood vessels. Collagen type I, in addition to its mechanical function, has a signaling function and is involved in the organization of the extracellular matrix, which, in turn, affects the organization of the epidermis and dermis [23]. Cells are able to directly bind to collagen monomers through integrins α1β1, α2β1, α10β1, and α11β1. Furthermore, this complex is involved in cell signaling, cell adhesion, cell migration, and remodeling of the collagen matrix [24].
Collagen type III is the main collagen in the skin of the fetus, while less is found in the adult skin. Furthermore, it is present in the reticular organs and the walls of blood vessels, and it is often found in fibers together with type I collagen fibrils [25]. Collagen type III is the most important in hollow organs, but it also interacts with platelets during blood coagulation (through specific glycoproteins and non-integrin receptors) and plays an important role as a signaling molecule in tissue regeneration (by participating in cellular adhesion, migration, proliferation, and differentiation through interaction with receptors on the cell surface, including integrins) [26]. Throughout a person’s life, the ratio of collagen types I and III fibers shifts towards an increase in collagen type I [27].
The COL3A1 gene is tail-to-tail with another fibrillar collagen gene, COL5A2; hence they are believed to have evolved from the same ancestor [28].
Collagen type V plays a valuable regulatory role and is found in soft tissues, placenta, blood vessels, and chorion. Without collagen type V, the assembly of the fibrillar type I collagen fiber is not possible. Collagen type V is located in the region of the N-terminal domain on the fibril surface and is therefore believed to determine the site of the beginning of fibril assembly in vivo [29].
Collagen types IV and VII and laminin form the basis of the epidermal basement membrane, providing anchoring sites (anchoring of endothelial cells and keratinocytes) and performing barrier functions in the epidermis [30]. Collagen type IV belongs to the group of network-forming collagens and is part of the basement membrane and lens capsule [31]. It belongs to collagens that form filament beads and can be found in microfibrils in soft tissues and cartilage [32].
Collagen type VII belongs to the group of collagens that form anchor fibrils located in the dermo-epidermal junction and is responsible for the strength of this junction [33]. Collagen type XIV belongs to fibril-associated collagens and is common for various soft tissues; it interacts with the surface of fibrils, regulating fibrillogenesis [34]. Collagen type XVII belongs to transmembrane collagens and is located on the surface of epidermal cells. It is a component of hemidesmosomes (multi-protein complexes located on the basement membrane), which mediate the attachment of keratinocytes to the underlying membrane [35].
Table 1. Genes responsible for the structure of collagen fibers, their activity, and diseases caused by their mutations (adapted from [36]).
| Gene (Protein) |
Chromosome Localization | Clinical Manifestations of Mutation/Polymorphism | Expression in the Skin (RPKM) |
|---|---|---|---|
| Fibrillar Collagens | |||
| COL1A1 (α1 chain of collagen type I) |
17q21.33 (51 exons) |
Osteogenesis imperfecta, classic type of Ehlers-Danlos syndrome, Caffey disease, idiopathic osteoporosis | 164.508 ± 48.747 |
| COL1A2 (α2 chain of collagen type I) |
17q21.3 (52 exons) |
Osteogenesis imperfecta, type VII B of Ehlers-Danlos syndrome, idiopathic osteoporosis, atypical Marfan syndrome | 190.333 ± 32.009 |
| COL3A1 (α1 chain of collagen type III) |
2q32.2 (51 exons) |
Ehlers-Danlos syndrome type IV, aortic and arterial aneurysms |
168.586 ± 46.57 |
| COL5A1 (α1 chain of collagen type V) |
9q34.3 (67 exons) |
Ehlers-Danlos syndrome types I and II | 7.679 ± 0.808 |
| COL5A2 (α2 chain of collagen type V) |
2q32.2 (55 exons) |
Ehlers-Danlos syndrome types I and II | 6.217 ± 1.778 |
| Networking Collagens | |||
| COL4A1 (α1 chain of collagen type IV) |
13q34 (54 exons) |
Cerebrovascular diseases, kidney and muscle pathology |
1.798 ± 0.45 |
| COL4A2 (6 subunits of collagen type IV) |
13q34 (48 exons) |
Cerebrovascular diseases, kidney and muscle pathology |
5.245 ± 1.325 |
| Collagens Forming Filament Beads | |||
| COL6A1 (α1 chain of collagen type VI) |
21q22.3 (35 exons) |
Bethlem myopathy | 65.872 ± 35.541 |
| COL6A2 (α2 chain of collagen type VI) |
21q22.3 (30 exons) |
Bethlem myopathy, Ullrich scleroatonic muscular dystrophy Bethlem myopathy |
68.022 ± 43.357 |
| COL6A3 (α3 chain of collagen type VI) |
2q37.3 (50 exons) |
Ullrich scleroatonic muscular dystrophy, autosomal dominant proximal myopathy |
68.022 ± 43.357 |
| COL6A5 (a protein that can interact with the α1 and α2 chains of type VI collagen to form a trimer) |
3q22.1 (44 exons) |
Eczema | 1.896 ± 0.958 |
| COL6A6 (a protein that regulates the interaction of epithelial cells with fibronectin) |
3q22.1 (44 exons) |
Dermatoses (eczema) | 0.183 ± 0.082 |
| Collagens Forming Anchor Fibrils | |||
| COL7A1 (α3 chain of collagen type VII) |
3q21.1 (120 exons) | Dystrophic epidermolysis bullosa | 62.83 ± 31.474 |
| Fibril-Associated Collagens | |||
| COL14A1 (α-chain of collagen type XIV) |
8q24.12 (50 exons) |
Increased risk of carcinogenesis | 3.173 ± 1.431 |
| Transmembrane Collagens | |||
| COL17A1 (α1 chain of collagen type XVII) |
10q25.1 (56 exons) |
Generalized atrophic epidermolysis and epidermolysis bullosa |
284.358 ± 48.16 |
The data on the role of collagen genes under physiological conditions presented in Table 1 confirm that genes encoding fibrillar collagens (collagen types I and III) and collagen type XVII have the highest expression in the skin. In the case of skin damage and activation of regeneration processes in fibroblasts, the highest expression of the COL1A1, COL1A2, and COL3A1 genes is also observed [37]. The gene expression of other types of collagens has only been detected in trace amounts, since they are not structural proteins of the skin. These genes are responsible for the primary polypeptide sequence of amino acids in the collagen molecule, and their mutations, single nucleotide variants (SNVs), and polymorphisms can lead to a disruption in the amino acid sequence of collagen molecules with subsequent disruption of protein function.
Not all mutations and/or SNVs lead to the formation of a pathological protein [38], but for each candidate gene, there are databases of pathological mutations, SNVs, and their contribution to the development of various human diseases [39].
Monogenic diseases caused by mutations in the collagen genes (Table 1) have a relatively low population frequency, while multi-factorial pathologies are widespread [40]. In these pathologies, additional adverse environmental factors are required for the genetic defect in a candidate gene (SNVs or polymorphisms) to be manifested as a pathological phenotype. In Russia, such collagen fiber pathologies are known as a group of connective tissue dysplasias. In contrast, elsewhere in the world, there is no single term; different studies show the contribution of SNVs or polymorphisms to the development of various (in terms of clinical course and prognosis) multi-factorial syndromes of collagen formation impairment, including in the skin.
3. Epigenetic Regulation of Collagen Synthesis
Epigenetics studies the inherited changes in protein synthesis that are not due to changes in the nucleotide sequence. Typically, such changes are caused by regulators of protein synthesis—methylation and demethylation of DNA, acetylation and deacetylation of histones, phosphorylation and dephosphorylation of transcription factors, and the action of regulatory microRNA (miRNA)—and other intracellular mechanisms. Modification of DNA and histones (which are involved in DNA packaging in the nucleus cells) alters the histone-histone and histone-DNA interactions, regulating the availability of transcription factors and influencing gene transcription [41]. Among other factors, the modification of epigenetic mechanisms underlies the mechanisms of skin and collagen fiber aging.
The roles of DNA and histone methylation and histone acetylation are the most studied [42]. In particular, DNA methylation results in transcription repression and the long-term maintenance of genome stability; however, in some sporadic cases, DNA methylation leads to the activation of genes in several types of cells [43]. Demethylation of DNA is facilitated by the influence of certain external and internal factors. Maintaining methylated DNA is important for the preservation of progenitor cells and skin self-renewal [44].
With skin aging, so-called epigenetic drift accumulates, and, as a result, both hypomethylated and hypermethylated DNA regions accumulate. Furthermore, ultraviolet (UV) radiation makes a large contribution to DNA hypomethylation, and the degree of hypomethylation correlates with clinical indicators of skin photoaging [45]. An example of epigenetic changes is a decrease in the regulation of the gene encoding LOX in old fibroblasts, as a result of which the mechanical properties of the skin are reduced [46].
Methylation of histones, depending on which site is modified, can lead to the activation or suppression of transcription.
The acetylation (deacetylation) of histone tails has the opposite effect of methylation (demethylation). Specifically, acetylation leads to the relaxation of chromatin and the activation of transcription. Deacetylation, on the contrary, leads to denser curling of chromatin and inhibition of transcription.
As a result of their participation in histone acetylation, specific NAD+-dependent enzymes (sirtuins) (SIRTs) play a key role in epigenetic regulation and facilitate transcription. Moreover, they are involved in the control of energy metabolism and oxidative stress, cell survival, response to UV damage, DNA repair, tissue regeneration, and inflammation [47]. In the dermis, sirtuins can inhibit collagen degradation, regulate DNA repair, and increase the activity of collagen type I synthesis by fibroblasts. The activity of sirtuins decreases with age and under oxidative stress conditions [48].
4. Conclusions
A large number of genetic and epigenetic factors affect the functioning of collagen fibers and, accordingly, the mechanical properties of the skin. Mutations of the genes that encode collagen proteins, MMP, and/or glycosaminoglycans, also the enzymes involved in post-translational collagen modifications, are causes of various forms of collagenopathies in humans [49].
The functioning and degeneration processes of collagen fibers in the skin are genetically determined. Therefore, mutations in fibrillar skin collagens lead to hereditary diseases, such as osteogenesis imperfecta, Ehlers-Danlos syndrome, Kaffi’s disease, idiopathic osteoporosis, etc. Furthermore, mutations in non-fibrillar skin collagens lead to cerebrovascular diseases, kidney and muscle pathologies, and epidermolysis bullosa. In Russian medicine, the terms differentiated and undifferentiated hereditary connective tissue dysplasias were previously proposed. The introduction of modern methods of molecular genetic diagnostics indicates that the most common hereditary (“differentiated”) collagenopathies include osteogenesis imperfecta, Ehlers-Danlos syndrome, Kaffi’s disease, and Marfan syndrome [50], which should be taken into account by doctors of aesthetic medicine. These are monogenic syndromes of Mendelian inheritance, caused by causal (pathogenic) gene mutations, in which the contribution of the environment is minimal or absent. For example, the genes involved in the development of Ehlers-Danlos syndrome include COL5A1, COL5A2, COL3A1, PLOD1, COL1A1, COL1A2, ADAMTS2, TNXB, FMNA, CHST14, SLC39A13, B4GALT7, and FKBP14 [25].
However, the number of associative genetic studies concerning multi-factorial (“undifferentiated”) collagenopathies is increasing. In these diseases, both the carriage of polymorphisms in candidate collagen genes and the influence of environmental factors are important. This is the reason for the higher incidence of multi-factorial collagenopathies in the population as compared with monogenic collagenopathies, many of which are rare (orphan). Investigations of the contribution of SNVs and polymorphisms to the development of multi-factorial connective tissue diseases, in general, and to the development of human skin collagen pathologies, in particular [39], are relevant.
However, associative genetic studies concerning the genes responsible for collagen fiber function are currently insufficient to compile a complete and clear personalized algorithm for the management of such patients by cosmetologists and dermatologists. Therefore, doctors, to a greater extent, are guided by the clinical picture, i.e., increased flabbiness, hyper-elasticity, early manifestation of aging, and other signs indirectly indicating a pathology in the collagen link. Therefore, only on the basis of the clinical picture can’t doctors form a treatment plan aimed at protecting and improving the synthesis of collagen fibers. Such recommendations, based on external and internal factors, might include, for example, lifestyle changes, additional intake of nutrients (vitamins, minerals), and mesotherapy (bio-revitalization) with amino acids and cofactors necessary for collagen synthesis. Implementing the results of molecular genetic diagnostics of monogenic and multi-factorial collagenopathies and their translation into real cosmetic, dermatologic, and plastic surgery practice will increase the efficiency and safety of local and general therapies for normal and pathological skin aging processes. The importance of translating the results of basic research into real clinical practice has been confirmed by research in recent years. For example, various studies [35][39][51][52][53] demonstrated the contribution of diverse factors related to the skin, opening up potential opportunities for therapeutic interventions with various cosmetic ingredients.
References
- Kapuler, O.; Selskaya, B.; Galeeva, A.; Kamilo, F. Metabolism of collagen fibers in the presence of age-related changes. Vrach 2015, 8, 64–69.
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014, 20, 683–696.
- Manturova, N.E.; Stenko, A.G.; Petinati, Y.A.; Chaikovskaya, E.A.; Bolgarina, A.A. Injectable collagen in correction of age-related skin changes: Experimental and clinical parallels. Bull. RSMU 2019, 1, 79–85.
- Blair, M.J.; Jones, J.D.; Woessner, A.E.; Quinn, K.P. Skin structure-function relationships and the wound healing response to intrinsic aging. Adv. Wound Care (New Rochelle) 2020, 9, 127–143.
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738.
- Goh, K.L.; Holmes, D.F. Collagenous extracellular matrix biomaterials for tissue engineering: Lessons from the common sea urchin tissue. Int. J. Mol. Sci. 2017, 18, 901.
- Seo, B.R.; Chen, X.; Ling, L.; Song, Y.H.; Shimpi, A.A.; Choi, S.; Gonzalez, J.; Sapudom, J.; Wang, K.; Andresen Eguiluz, R.C.; et al. Collagen microarchitecture mechanically controls myofibroblast differentiation. Proc. Natl. Acad. Sci. USA 2020, 117, 11387–11398.
- Park, S.; Jung, W.H.; Pittman, M.; Chen, J.; Chen, Y. The effects of stiffness, viscosity, and geometry of microenvironment in Homeostasis, Aging and Diseases. J. Biomech. Eng. 2020, 142, 100804.
- Roy, B.; Yuan, L.; Lee, Y.; Bharti, A.; Mitra, A.; Shivashankar, G.V. Fibroblast rejuvenation by mechanical reprogramming and redifferentiation. Proc. Natl. Acad. Sci. USA 2020, 117, 10131–10141.
- Litman, T. Personalized medicine-concepts, technologies, and applications in inflammatory skin diseases. APMIS 2019, 127, 386–424.
- Pawlaczyk, M.; Lelonkiewicz, M.; Wieczorowski, M. Age-dependent biomechanical properties of the skin. Postepy Dermatol. Alergol. 2013, 30, 302–306.
- Vashi, N.A.; de Castro Maymone, M.B.; Kundu, R.V. Aging differences in ethnic skin. J. Clin. Aesthetic Dermatol. 2016, 9, 31–38.
- Lynch, B.; Bonod-Bidaud, C.; Ducourthial, G.; Affagard, J.S.; Bancelin, S.; Psilodimitrakopoulos, S.; Ruggiero, F.; Allain, J.M.; Schanne-Klein, M.C. How aging impacts skin biomechanics: A multiscale study in mice. Sci. Rep. 2017, 7, 13750.
- Shao, Y.; Qin, Z.; Alexander Wilks, J.; Balimunkwe, R.M.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Physical properties of the photodamaged human skin dermis: Rougher collagen surface and stiffer/harder mechanical properties. Exp. Dermatol. 2019, 28, 914–921.
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833.
- Yang, W.; Sherman, V.R.; Gludovatz, B.; Schaible, E.; Stewart, P.; Ritchie, R.O.; Meyers, M.A. On the tear resistance of skin. Nat. Commun. 2015, 6, 6649.
- Mostaço-Guidolin, L.; Rosin, N.L.; Hackett, T.L. Imaging Collagen in Scar Tissue: Developments in Second Harmonic Generation Microscopy for Biomedical Applications. Int. J. Mol. Sci. 2017, 18, 1772.
- de Wild, M.; Pomp, W.; Koenderink, G.H. Thermal memory in self-assembled collagen fibril networks. Biophys. J. 2013, 105, 200–210.
- Terzi, A.; Storelli, E.; Bettini, S.; Sibillano, T.; Altamura, D.; Salvatore, L.; Madaghiele, M.; Romano, A.; Siliqi, D.; Ladisa, M.; et al. Effects of processing on structural, mechanical and biological properties of collagen-based substrates for regenerative medicine. Sci. Rep. 2018, 8, 1429.
- Kang, M.C.; Yumnam, S.; Kim, S.Y. Oral intake of collagen peptide attenuates ultraviolet B irradiation-induced skin dehydration in vivo by regulating hyaluronic acid synthesis. Int. J. Mol. Sci. 2018, 19, 3551.
- Söderhäll, C.; Marenholz, I.; Kerscher, T.; Rüschendorf, F.; Esparza-Gordillo, J.; Worm, M.; Gruber, C.; Mayr, G.; Albrecht, M.; Rohde, K.; et al. Variants in a novel epidermal collagen gene (COL29A1) are associated with atopic dermatitis. PLoS Biol. 2007, 5, e242.
- Manka, S.W.; Bihan, D.; Farndale, R.W. Structural studies of the MMP-3 interaction with triple-helical collagen introduce new roles for the enzyme in tissue remodeling. Sci. Rep. 2019, 9, 18785.
- Ouyang, M.; Yu, J.Y.; Chen, Y.; Deng, L.; Guo, C.L. Cell-extracellular matrix interactions in the fluidic phase direct the topology and polarity of self-organized epithelial structures. Cell Prolif. 2021, 54, e13014.
- Musiime, M.; Chang, J.; Hansen, U.; Kadler, K.E.; Zeltz, C.; Gullberg, D. Collagen Assembly at the Cell Surface: Dogmas Revisited. Cells 2021, 10, 662.
- Kuivaniemi, H.; Tromp, G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene 2019, 707, 151–171.
- Wulandari, E.; Jusman, S.W.; Moenadjat, Y.; Jusuf, A.A.; Sadikin, M. Expressions of collagen I and III in hypoxic keloid tissue. Kobe J. Med. Sci. 2016, 62, E58–E69.
- Kehlet, S.N.; Willumsen, N.; Armbrecht, G.; Dietzel, R.; Brix, S.; Henriksen, K.; Karsdal, M.A. Age-related collagen turnover of the interstitial matrix and basement membrane: Implications of age- and sex-dependent remodeling of the extracellular matrix. PLoS ONE 2018, 13, e0194458.
- Niu, K.; Chen, X.; Lu, Y. COL3A1 rs1800255 polymorphism is associated with pelvic organ prolapse susceptibility in Caucasian individuals: Evidence from a meta-analysis. PLoS ONE 2021, 16, e0250943.
- Xu, X.; Wang, Z.; Zan, T. A case of Ehlers-Danlos syndrome presenting with widened atrophic scars of forehead, elbow, knee, and pretibial area: A case report. Medicine (Baltimore) 2019, 98, e17138.
- Costa, A.; Naranjo, J.D.; Londono, R.; Badylak, S.F. Biologic Scaffolds. Cold Spring Harb. Perspect. Med. 2017, 7, a025676.
- Brown, K.L.; Cummings, C.F.; Vanacore, R.M.; Hudson, B.G. Building collagen IV smart scaffolds on the outside of cells. Protein Sci. 2017, 26, 2151–2161.
- Fidler, A.L.; Boudko, S.P.; Rokas, A.; Hudson, B.G. The triple helix of collagens—An ancient protein structure that enabled animal multicellularity and tissue evolution. J. Cell Sci. 2018, 131, jcs203950.
- Gatseva, A.; Sin, Y.Y.; Brezzo, G.; Van Agtmael, T. Basement membrane collagens and disease mechanisms. Essays Biochem. 2019, 63, 297–312.
- Li, L.; Sun, Z.; Chen, J.; Zhang, Y.; Shi, H.; Zhu, L. Genetic polymorphisms in collagen-related genes are associated with pelvic organ prolapse. Menopause 2020, 27, 223–229.
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350.
- NCBI. Genes & Expression. Gene. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 23 April 2021).
- Wietecha, M.S.; Pensalfini, M.; Cangkrama, M.; Müller, B.; Jin, J.; Brinckmann, J.; Mazza, E.; Werner, S. Activin-mediated alterations of the fibroblast transcriptome and matrisome control the biomechanical properties of skin wounds. Nat. Commun. 2020, 11, 2604.
- Demina, O.M.; Karpova, E.I.; Borzykh, O.B. Modern aspects of medical genetics. Russ. J. Clin. Dermatol. Venereol. 2021, 20, 124–134.
- Borzykh, O.B.; Petrova, M.M.; Shnayder, N.A.; Nasyrova, R.F. Problems of implementation of personalized medicine in medical cosmetology in Russia. Sib. Med. Rev. 2021, 2, 12–22.
- Yeo, J.; Jung, G.; Tarakanova, A.; Martín-Martínez, F.J.; Qin, Z.; Cheng, Y.; Zhang, Y.W.; Buehler, M.J. Multiscale modeling of keratin, collagen, elastin and related human diseases: Perspectives from atomistic to coarse-grained molecular dynamics simulations. Extreme Mech. Lett. 2018, 20, 112–124.
- Yang, I.V. Epigenomics of idiopathic pulmonary fibrosis. Epigenomics 2012, 4, 195–203.
- Perdigoto, C.N.; Valdes, V.J.; Bardot, E.S.; Ezhkova, E. Epigenetic regulation of epidermal differentiation. Cold Spring Harb. Perspect. Med. 2014, 4, a015263.
- Stoll, S.; Wang, C.; Qiu, H. DNA methylation and histone modification in hypertension. Int. J. Mol. Sci. 2018, 19, 1174.
- Vandiver, A.R.; Irizarry, R.A.; Hansen, K.D.; Garza, L.A.; Runarsson, A.; Li, X.; Chien, A.L.; Wang, T.S.; Leung, S.G.; Kang, S.; et al. Age and sun exposure-related widespread genomic blocks of hypomethylation in nonmalignant skin. Genome Biol. 2015, 16, 80.
- Moulin, L.; Cenizo, V.; Antu, A.N.; André, V.; Pain, S.; Sommer, P.; Debret, R. Methylation of LOXL1 promoter by DNMT3A in aged human skin fibroblasts. Rejuvenation Res. 2017, 20, 103–110.
- Ghosh, K.; O’Neil, K.; Capell, B.C. Histone modifiers: Dynamic regulators of the cutaneous transcriptome. J. Dermatol. Sci. 2018, 89, 226–232.
- Garcia-Peterson, L.M.; Wilking-Busch, M.J.; Ndiaye, M.A.; Philippe, C.G.A.; Setaluri, V.; Ahmad, N. Sirtuins in skin and skin cancers. Skin Pharmacol. Physiol. 2017, 30, 216–224.
- Carlomosti, F.; D’Agostino, M.; Beji, S.; Torcinaro, A.; Rizzi, R.; Zaccagnini, G.; Maimone, B.; Di Stefano, V.; De Santa, F.; Cordisco, S.; et al. Oxidative stress-induced miR-200c disrupts the regulatory loop among SIRT1, FOXO1, and eNOS. Antioxid. Redox Signal. 2017, 27, 328–344.
- Lu, Y.; Zhang, S.; Wang, Y.; Ren, X.; Han, J. Molecular mechanisms and clinical manifestations of rare genetic disorders associated with type I collagen. Intractable Rare Dis. Res. 2019, 8, 98–107.
- Castori, M. Ehlers-Danlos syndrome, hypermobility type: An underdiagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations. ISRN Dermatol. 2012, 2012, 751768.
- Nyström, A.; Thriene, K.; Mittapalli, V.; Kern, J.S.; Kiritsi, D.; Dengjel, J.; Bruckner-Tuderman, L. Losartan ameliorates dystrophic epidermolysis bullosa and uncovers new disease mechanisms. EMBO Mol. Med. 2015, 7, 1211–1228.
- Sotiropoulou, G.; Zingkou, E.; Pampalakis, G. Redirecting drug repositioning to discover innovative cosmeceuticals. Exp. Dermatol. 2021, 30, 628–644.
- Shnayder, N.A.; Dyuzhakova, A.V.; Vaiman, E.E.; Nikitina, E.I.; Borzykh, O.B.; Nasyrova, R.F. The role of genetic factors of endogenous hyaluronic acid metabolism in maintaining skin homeostasis. Bull. Dermatol. Venerol. 2021, 97, 24–38.
More
Information
Subjects:
Dermatology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Entry Collection:
Peptides for Health Benefits
Revisions:
2 times
(View History)
Update Date:
21 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




