Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ding, H. Pigeon Muscle Development. Encyclopedia. Available online: https://encyclopedia.pub/entry/13086 (accessed on 08 February 2026).
Ding H. Pigeon Muscle Development. Encyclopedia. Available at: https://encyclopedia.pub/entry/13086. Accessed February 08, 2026.
Ding, Hao. "Pigeon Muscle Development" Encyclopedia, https://encyclopedia.pub/entry/13086 (accessed February 08, 2026).
Ding, H. (2021, August 12). Pigeon Muscle Development. In Encyclopedia. https://encyclopedia.pub/entry/13086
Ding, Hao. "Pigeon Muscle Development." Encyclopedia. Web. 12 August, 2021.
Copy Citation
The growth and development of skeletal muscle determine the meat production performance of pigeons and are regulated by complex gene networks. To explore the genes involved in regulating the growth and development of pigeon skeletal muscle, RNA sequencing (RNA−seq) was used to characterise gene expression profiles during the development and growth of pigeon breast muscle and identify differentially expressed genes (DEGs) among different stages.
pigeon
skeletal muscle
development and growth
1. Introduction
Pigeon meat is rich in nutrition, high in protein, low in fat, and high in medicinal value. In China, pigeon meat is called “animal ginseng” and is considered an advanced nourishment product that is increasingly favoured by consumers [1]. Meat production performance is an important index to measure the economic value of pigeons. However, the genetic improvement of the meat production performance of pigeons is relatively lagging in comparison with other poultry. The growth and development of skeletal muscle determine the meat production performance of pigeons [2]. Therefore, understanding the molecular regulation mechanism of pigeon skeletal muscle growth and development is an important prerequisite for improving meat production performance by molecular breeding technology [3].
The development of skeletal muscle is closely related to skeletal muscle cell differentiation. Myogenic regulatory factor family (MRF) members play essential roles in the process of skeletal muscle cell differentiation, including MyoD (myogenic determining factor), MyoG (myogenin), MRF4 (myogenic regulatory factor 4), and Myf5 (myogenic factor 5) [4]. However, the process of skeletal muscle growth and development involves multi−gene expression, signal transduction, and network regulation, and there are still a large number of regulatory factors to be identified.
RNA sequencing (RNA−seq) can explore the differences of gene types and expression levels at the overall level and directly link the changes of gene expression levels with phenotypic changes. In recent years, to explore the molecular mechanism of critical economic traits, considerable transcriptome studies on livestock and poultry were carried out using RNA−seq technology [5]. Xing et al. revealed essential genes related to muscle fat and abdominal fat deposition in chickens during the development process and identified 21 key genes in total, using RNA−Seq analysis [6]. Hu et al. identified several genes and pathways that may regulate skeletal muscle growth in the black Muscovy duck using RNA−seq [7]. RNA−seq technology has also been applied to pigeon transcriptome analysis. Wang et al. performed a comprehensive investigation into miRNA transcriptomes in livers across three pigeon developmental stages using RNA−seq and identified several vital target genes (e.g., TNRC6B, FRS2, PTCH1, etc.) of DE miRNA, which is closely linked to liver development [8]. Ye et al. compared the transcriptomes of muscle and liver tissues between squabs of two breeds to identify candidate genes associated with the differences in the capacity of fat deposition. A total of 27 genes were identified as a basis for further investigations to screen markers closely associated with intramuscular fat content and fatty acid composition in squabs [9].
2. Weighted Gene Co−Expression Network Analysis
To investigate the relationship between DEGs and muscle development and growth, we performed a WGCNA analysis based on the expression of 11,311 DEGs. WGCNA categorised 11,311 DEGs into 12 modules with a soft power of 5 (Figure 1A,B). Among the 12 modules, black and brown were significantly correlated with day 1 and 10 stages, while turquoise and cyan were significantly correlated with embryonic development stages of days 8 and 13 (r > 0.4) (Figure 1C).
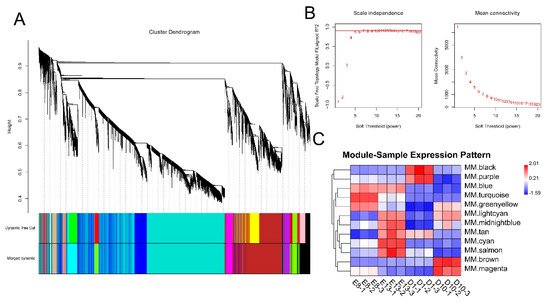
Figure 1. WGCNA analysis of DEGs. (A) Clustering dendrogram of DEGs with dissimilarity based on the topological overlap. Dynamic tree cutting was applied to identify modules by dividing the dendrogram at significant branch points. Modules are displayed with different colours in the horizontal bar immediately below the dendrogram. (B) Determination of soft−thresholding power for WGCNA analysis. The graph indicates that soft thresholding power above 5 meets scale−free topology above 0.85. (C) Heatmap of the correlation between samples and gene modules. Each row corresponds to a module eigengene, each column to a sample. Each cell contains the corresponding correlation value. Red and blue colours represent positive and negative correlations, respectively. The darker the colour, the higher the correlation.
3. Visualisation of the Pigeon Muscle Development and Growth−Related Modules
To further explore candidate DEGs that regulate pigeon skeletal muscle growth and development, we visualised the four significant modules identified by WGCNA based on the top 1000 mRNA−mRNA interaction pairs in each module. Four mRNA−mRNA regulatory networks were constructed, corresponding to black, brown, cyan, and turquoise modules, respectively. The black, brown, cyan and turquoise modules contained 265, 308, 276, and 402 mRNAs, respectively (Figure 2).
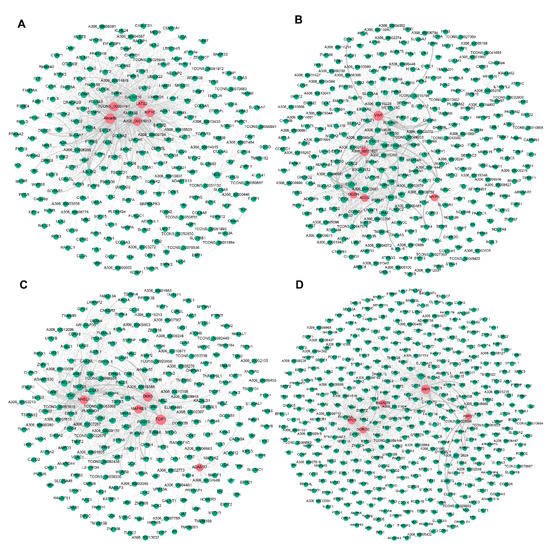
Figure 2. Visualisation of the four modules related to pigeon muscle development and growth. Networks (A–D) represent visualisations of gene interactions of black, brown, cyan and turquoise modules, respectively. The node size indicates the node degree. The line thickness indicates the strength of the correlation. Red nodes represent hub DEGs in each module.
References
- Chen, M. The Growth and Nutrition Regulation of Domestic Pigeon (Columba livia). Poult. Sci. 2019, 8, 49–52.
- Guo, L.; Huang, W.; Chen, B.; Jebessa Bekele, E.; Chen, X.; Cai, B.; Nie, Q. gga−mir−133a−3p Regulates Myoblasts Proliferation and Differentiation by Targeting PRRX1. Front. Genet. 2018, 9, 577.
- Xu, K.; Han, C.X.; Zhou, H.; Ding, J.M.; Xu, Z.; Yang, L.Y.; He, C.; Akinyemi, F.; Zheng, Y.M.; Qin, C.; et al. Effective MSTN Gene Knockout by AdV−Delivered CRISPR/Cas9 in Postnatal Chick Leg Muscle. Int. J. Mol. Sci. 2020, 21, 2584.
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233.
- Costa, V.; Angelini, C.; De Feis, I.; Ciccodicola, A. Uncovering the complexity of transcriptomes with RNA−Seq. J. Biomed. Biotechnol. 2010, 2010, 853916.
- Xing, S.; Liu, R.; Zhao, G.; Liu, L.; Groenen, M.A.M.; Madsen, O.; Zheng, M.; Yang, X.; Crooijmans, R.; Wen, J. RNA−Seq Analysis Reveals Hub Genes Involved in Chicken Intramuscular Fat and Abdominal Fat Deposition During Development. Front. Genet. 2020, 11, 1009.
- Hu, Z.; Cao, J.; Liu, G.; Zhang, H.; Liu, X. Comparative Transcriptome Profiling of Skeletal Muscle from Black Muscovy Duck at Different Growth Stages Using RNA−seq. Genes 2020, 11, 1228.
- Wang, X.; Yan, P.; Liu, L.; Luo, Y.; Zhao, L.; Liu, H.; Tang, Q.; Long, K.; Jin, L.; Ma, J.; et al. MicroRNA expression profiling reveals potential roles for microRNA in the liver during pigeon (Columba livia) development. Poult. Sci. 2020, 99, 6378–6389.
- Ye, M.; Zhou, B.; Wei, S.; Ding, M.; Lu, X.; Shi, X.; Ding, J.; Yang, S.; Wei, W. Transcriptomic Analysis Identifies Candidate Genes Related to Intramuscular Fat Deposition and Fatty Acid Composition in the Breast Muscle of Squabs (Columba). G3 Bethesda Md. 2016, 6, 2081–2090.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
893
Revisions:
2 times
(View History)
Update Date:
12 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




