
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adelaide Fernandes | + 513 word(s) | 513 | 2020-08-20 04:53:40 | | | |
| 2 | Conner Chen | + 340 word(s) | 853 | 2020-08-25 11:37:17 | | |
Video Upload Options
Multiple sclerosis (MS) is the most common autoimmune and demyelinating disease of the central nervous system (CNS), characterized, in the majority of cases, by initial relapses that later evolve into progressive neurodegeneration, severely impacting patients’ motor and cognitive functions. Despite the availability of immunomodulatory therapies effective to reduce relapse rate and slow disease progression, they all failed to restore CNS myelin that is necessary for MS full recovery. Microglia are the primary inflammatory cells present in MS lesions, therefore strongly contributing to demyelination and lesion extension. Thus, many microglial-based therapeutic strategies have been focused on the suppression of microglial pro-inflammatory phenotype and neurodegenerative state to reduce disease severity.
1. Introduction
Microglia, the resident immune cells and macrophages of the CNS, make up only 15% of the total brain cells [1] and 10% of whole glial cells [2][3]. Nonetheless, due to their essential functions as immune mediators and primary phagocytes of the brain and spinal cord, microglia have attracted much attention and research. This yolk sac-derived population colonizes the brain during embryogenesis and differentiates under the influence of CNS microenvironment [4], residing in the healthy nervous system as a highly stable population with long and ramified processes interacting with blood vessels, neurons, and other glial cells in a dynamic “surveillant state” [5]. “Surveillant” microglia maintain CNS homeostasis by sensing pathologic events, scavenging pathogen-associated molecular patterns (PAMPs)/danger-associated molecular patterns (DAMPs), and by phagocytosing dead cells and misfolded proteins [6][7]. At the same time, in physiological conditions, they are also engaged in the regulation of biological processes either remodeling synaptic plasticity [8][9], supporting neuronal survival [10][33], brain sexual differentiation [11][12], or promoting proper myelination [13][14][15].
2. Microglial Phagocytosis
New compounds, in non-clinical testing, revealed some encouraging effects regarding microglial phagocytosis (Figure 1). To begin with, studies from Chen et al. found that the incubation of primary microglia with two omega-3 polyunsaturated fatty acids-docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)—modulated the cell phenotype towards a more regenerative state and enhanced myelin phagocytosis in vitro. Attractively, such treatment in vivo reduced demyelination and ameliorated motor and cognitive functions in a cuprizone demyelinating model [16]. Additionally encouraging, a human recombinant IgM antibody (rHIgM22) effectively modulated microglial myelin phagocytosis. Indeed, in vitro testing showed that rHIgM22 could bind to myelin, tagging debris for their internalization by microglia, in a complement-mediated manner [17], and, when administrated in vivo, rHIgM22 promoted OPC differentiation, accelerated remyelination, and even ameliorated memory deficits in rodent models of cuprizone and chronic virus-induced demyelination [18][19][20]. Giving this newly association between phagocytosis and microglial neuroprotective functions over brain pathologies, two more studies concerning modulation of microglial phagocytosis have just been published. In the first one, the authors treated rat microglial cells with endocannabinoid 2-AG. By enhancing the mRNA levels of phagocytosis associated genes (cd206, sirp1α, msr1, and Trem2), endocannabinoid 2-AG boosted phagocytosis of both Coli-coated beads and rat-purified myelin debris. Moreover, using a mice model of MS, the Theiler’s induced demyelinating disease model (TMEV-IDD), TMEV-induced mice treated with endocannabinoid 2-AG showed augmented microglial myelin phagocytosis in the corpus callosum that likely resulted from the observed upregulation of msr1 and Lamp1 mRNA levels, genes associated with microglial phagocytic machinery and phagosome formation, respectively. Interestingly, these animals had increased OPC differentiation and remyelination following demyelination in the corpus callosum [21]. In the second study, Liu et al. observed that a new pharmacological treatment with pseudoginsenoside-F11 accelerated CR3-dependent myelin phagocytosis in a microglial culture after oxygen-glucose deprivation (OGD) and permanent middle cerebral artery occlusion (pMCAO) in vivo and, by doing so, decreased de infarct area and improved neurological functions in pMCAO-treated rats [22].
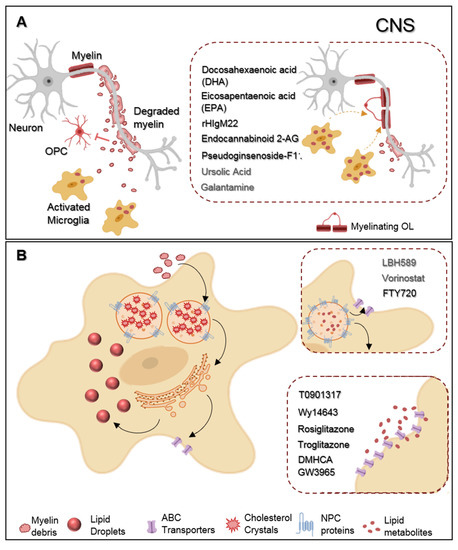
Figure 1. Representative scheme of microglia myelin phagocytosis modulating drugs. (A) During multiple sclerosis (MS) progression myelin is degraded into myelin-toxic debris within demyelinated plaques in the central nervous system (CNS). Giving debris toxicity towards oligodendrocyte (OL) precursor cells (OPCs) inhibiting their differentiation into full differentiated myelinating OLs, myelin debris must be cleared away by microglia. Regarding therapeutics, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) increase myelin uptake by microglia. Endocannabinoid 2-AG favors both myelin microglial clearance and OPC differentiation. Pseudoginsenoside-F11 accelerates CR3-dependent myelin phagocytosis while rHIgM22 binds to myelin debris and also facilitates their entrance towards microglia. Galantamine increases microglial uptake of Aβ aggregates and ursolic acid can interfere in the upstream regulation of CD36 expression, so that they may likely have an effect on myelin phagocytosis. (B) Demyelinated lesions are associated with foamy phagocytes presenting a pro-inflammatory profile as a result of over-internalization of cholesterol-rich myelin debris. Intracellularly, excessive cholesterol dysregulates lipid metabolism and efflux pathways and accumulates in the lysosomes forming cholesterol crystals or can be stored into lipid droplets. Proposed therapeutics are based on the functionalization of lipid metabolism and associated pathways. LBH589 and Vorinostat increase the expression of NPC proteins in fibroblasts, facilitating cholesterol release from lysosomes, for what we envision a possible effect on microglia as well. FTY720 treatment lead to the overexpression of both NPC and ABC transporters also promoting lipid efflux from human primary macrophages. DMHCA and GW3965 directly or Wy14643, Rosiglitazone, and Troglitazone, indirectly, activate the LXR/RXR heterodimeric transcription factor which upregulates its transcriptional targets ABC transporters then again facilitates cholesterol/lipid exit from macrophages.
References
- Cardona, A.E.; Huang, D.; Sasse, M.E.; Ransohoff, R.M. Isolation of murine microglial cells for RNA analysis or flow cytometry. Nat. Protoc. 2006, 1, 1947–1951, doi:10.1038/nprot.2006.327.
- Perry, V.H.; Nicoll, J.A.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201, doi:10.1038/nrneurol.2010.17.
- Soulet, D.; Rivest, S. Bone-marrow-derived microglia: Myth or reality? Curr. Opin. Pharmacol. 2008, 8, 508–518, doi:10.1016/j.coph.2008.04.002.
- Ling, E.; Penney, D.; Leblond, C. Use of carbon labeling to demonstrate the role of blood monocytes as precursors of the ‘Ameboid Cells’ present in the corpus callosum of postnatal rats. J. Comp. Neurol. 1980, 193, 631–657, doi:10.1002/cne.901930304.
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318.
- von Bernhardi, R.; Heredia, F.; Salgado, N.; Munoz, P. Microglia function in the normal brain. Adv. Exp. Med. Biol. 2016, 949, 67–92, doi:10.1007/978-3-319-40764-7_4.
- Hanisch, U.K.; Kettenmann, H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007, 10, 1387–1394, doi:10.1038/nn1997.
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R. 3rd; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013, 155, 1596–1609, doi:10.1016/j.cell.2013.11.030.
- Ji, K.; Akgul, G.; Wollmuth, L.P.; Tsirka, S.E. Microglia actively regulate the number of functional synapses. PLoS ONE 2013, 8, e56293, doi:10.1371/journal.pone.0056293.
- Ueno, M.; Fujita, Y.; Tanaka, T.; Nakamura, Y.; Kikuta, J.; Ishii, M.; Yamashita, T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 2013, 16, 543–551, doi:10.1038/nn.3358.
- Nelson, L.H.; Warden, S.; Lenz, K.M. Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain Behav. Immun. 2017, 64, 11–22, doi:10.1016/j.bbi.2017.03.010.
- VanRyzin, J.W.; Marquardt, A.E.; Argue, K.J.; Vecchiarelli, H.A.; Ashton, S.E.; Arambula, S.E.; Hill, M.N.; McCarthy, M.M. Microglial phagocytosis of newborn cells is induced by endocannabinoids and sculpts sex differences in juvenile rat social play. Neuron 2019, 102, 435–449.e6, doi:10.1016/j.neuron.2019.02.006.
- Nicholas, R.S.; Wing, M.G.; Compston, A. Nonactivated microglia promote oligodendrocyte precursor survival and maturation through the transcription factor NF-kappa B. Eur. J. Neurosci. 2001, 13, 959–967.
- Pasquini, L.A.; Millet, V.; Hoyos, H.C.; Giannoni, J.P.; Croci, D.O.; Marder, M.; Liu, F.T.; Rabinovich, G.A.; Pasquini, J.M. Galectin-3 drives oligodendrocyte differentiation to control myelin integrity and function. Cell Death Differ. 2011, 18, 1746–1756, doi:10.1038/cdd.2011.40.
- Hoyos, H.C.; Rinaldi, M.; Mendez-Huergo, S.P.; Marder, M.; Rabinovich, G.A.; Pasquini, J.M.; Pasquini, L.A. Galectin-3 controls the response of microglial cells to limit cuprizone-induced demyelination. Neurobiol. Dis. 2014, 62, 441–455, doi:10.1016/j.nbd.2013.10.023.
- Chen, S.; Zhang, H.; Pu, H.; Wang, G.; Li, W.; Leak, R.K.; Chen, J.; Liou, A.K.; Hu, X. n-3 PUFA supplementation benefits microglial responses to myelin pathology. Sci. Rep. 2014, 4, 7458, doi:10.1038/srep07458.
- Zorina, Y.; Stricker, J.; Caggiano, A.O.; Button, D.C. Human IgM antibody rHIgM22 promotes phagocytic clearance of myelin debris by microglia. Sci. Rep. 2018, 8, 9392, doi:10.1038/s41598-018-27559-y.
- Warrington, A.E.; Bieber, A.J.; Ciric, B.; Pease, L.; Van Keulen, V.P.; Rodriguez, M. A recombinant human IgM promotes myelin repair after a single, very low dose. J. Neurosci. Res. 2007, 85, 967–976.
- Mullin, A.P.; Cui, C.; Wang, Y.; Wang, J.; Troy, E.; Caggiano, A.O.; Parry, T.J.; Colburn, R.W.; Pavlopoulos, E. rHIgM22 enhances remyelination in the brain of the cuprizone mouse model of demyelination. Neurobiol. Dis. 2017, 105, 142–155, doi:10.1016/j.nbd.2017.05.015.
- Cui, C.; Wang, J.; Mullin, A.P.; Caggiano, A.O.; Parry, T.J.; Colburn, R.W.; Pavlopoulos, E. The antibody rHIgM22 facilitates hippocampal remyelination and ameliorates memory deficits in the cuprizone mouse model of demyelination. Brain Res. 2018, 1694, 73–86, doi:10.1016/j.brainres.2018.05.013.
- Mecha, M.; Yanguas-Casas, N.; Feliu, A.; Mestre, L.; Carrillo-Salinas, F.; Azcoitia, I.; Yong, V.W.; Guaza, C. The endocannabinoid 2-AG enhances spontaneous remyelination by targeting microglia. Brain Behav. Immun. 2019, 77, 110–126, doi:10.1016/j.bbi.2018.12.013.
- Liu, Y.; Wu, C.; Hou, Z.; Fu, X.; Yuan, L.; Sun, S.; Zhang, H.; Yang, D.; Yao, X.; Yang, J. Pseudoginsenoside-F11 accelerates microglial phagocytosis of myelin debris and attenuates cerebral ischemic injury through complement receptor 3. Neuroscience 2020, 426, 33–49, doi:10.1016/j.neuroscience.2019.11.010.




