The present manuscript provides a comprehensive review of microglial myelin phagocytosis and its involvement in MS development and repair.
Abstract
Multiple sclerosis (MS) is the most common autoimmune and demyelinating disease of the Central Nervous System (CNS), characterized, in the majority of cases, by initial relapses that later evolve into progressive neurodegeneration, severely impacting patients’ motor and cognitive functions. Despite the availability of immunomodulatory therapies effective to reduce relapse rate and slow disease progression, they all failed to restore CNS myelin -necessary for MS full recovery. Microglia are the primary inflammatory cells present in MS lesions, therefore strongly contributing to demyelination and lesion extension. Thus, many microglial-based therapeutic strategies have been focused on the suppression of microglial pro-inflammatory phenotype and neurodegenerative state to reduce disease severity. On the other hand, the contribution of myelin phagocytosis advocating the neuroprotective role of microglia in MS has been less explored. Indeed, despite the presence of functional oligodendrocyte precursor cells (OPCs), within lesioned areas, MS plaques fail to remyelinate as a result of the over-accumulation of myelin-toxic debris that must be cleared away by microglia. Dysregulation of this process has been associated with the impaired neuronal recovery and deficient remyelination. In line with this, here we provide a comprehensive review of microglial myelin phagocytosis and its involvement in MS development and repair. Alongside, we discuss the potential of phagocytic-mediated therapeutic approaches and encourage their modulation as a novel and rational approach to ameliorate MS-associated pathology.
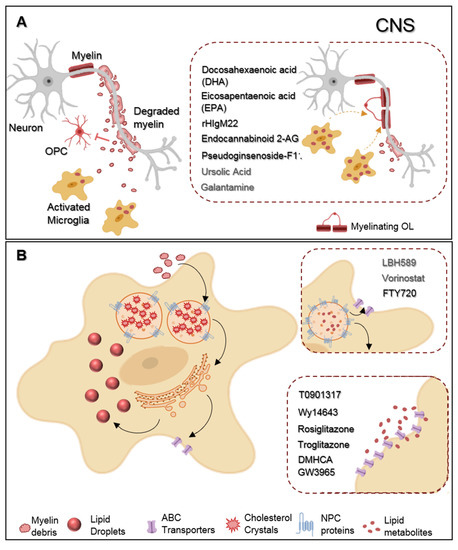
Figure. Representative scheme of microglia myelin phagocytosis modulating drugs. (A) During multiple sclerosis (MS) progression myelin is degraded into myelin-toxic debris within demyelinated plaques in the central nervous system (CNS). Giving debris toxicity towards oligodendrocyte (OL) precursor cells (OPCs) inhibiting their differentiation into full differentiated myelinating OLs, myelin debris must be cleared away by microglia. Regarding therapeutics, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) increase myelin uptake by microglia. Endocannabinoid 2-AG favors both myelin microglial clearance and OPC differentiation. Pseudoginsenoside-F11 accelerates CR3-dependent myelin phagocytosis while rHIgM22 binds to myelin debris and also facilitates their entrance towards microglia. Galantamine increases microglial uptake of Aβ aggregates and ursolic acid can interfere in the upstream regulation of CD36 expression, so that they may likely have an effect on myelin phagocytosis. (B) Demyelinated lesions are associated with foamy phagocytes presenting a pro-inflammatory profile as a result of over-internalization of cholesterol-rich myelin debris. Intracellularly, excessive cholesterol dysregulates lipid metabolism and efflux pathways and accumulates in the lysosomes forming cholesterol crystals or can be stored into lipid droplets. Proposed therapeutics are based on the functionalization of lipid metabolism and associated pathways. LBH589 and Vorinostat increase the expression of NPC proteins in fibroblasts, facilitating cholesterol release from lysosomes, for what we envision a possible effect on microglia as well. FTY720 treatment lead to the overexpression of both NPC and ABC transporters also promoting lipid efflux from human primary macrophages. DMHCA and GW3965 directly or Wy14643, Rosiglitazone, and Troglitazone, indirectly, activate the LXR/RXR heterodimeric transcription factor which upregulates its transcriptional targets ABC transporters then again facilitates cholesterol/lipid exit from macrophages.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21175960