Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Do Hyun Park | + 1411 word(s) | 1411 | 2021-07-13 04:37:03 | | | |
| 2 | Bruce Ren | -21 word(s) | 1390 | 2021-07-16 10:36:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Park, D.H. Painful Obstructive Chronic Pancreatitis Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/12088 (accessed on 07 February 2026).
Park DH. Painful Obstructive Chronic Pancreatitis Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/12088. Accessed February 07, 2026.
Park, Do Hyun. "Painful Obstructive Chronic Pancreatitis Treatment" Encyclopedia, https://encyclopedia.pub/entry/12088 (accessed February 07, 2026).
Park, D.H. (2021, July 15). Painful Obstructive Chronic Pancreatitis Treatment. In Encyclopedia. https://encyclopedia.pub/entry/12088
Park, Do Hyun. "Painful Obstructive Chronic Pancreatitis Treatment." Encyclopedia. Web. 15 July, 2021.
Copy Citation
There is limited evidence on the standard care for painful obstructive chronic pancreatitis (CP), while comparisons of endoscopic and surgical modes for pain relief have yielded conflicting results from small sample sizes.
pancreatitis
systematic review
meta-analysis
1. Introduction
Chronic pancreatitis is a prevailing health topic in the western countries, with a reported prevalence of around 50/100,000 persons [1][2]. Contributed by increasing societal affluence, alcohol consumption, and availability of diagnostic imaging, this condition is becoming more common also in the developing countries, ranging from 13.5 to 125 per 100,000 persons [3][4]. Alcohol is the single most common risk factor for chronic pancreatitis [1][5][6], and it predominantly affects men aged 40–60 years, imposing substantial socio-economic burdens. In the United Kingdom, it has been estimated that the direct and indirect costs relating to chronic pancreatitis totaled GBP 285.3 million per year [7]. Apart from alcoholic pancreatitis, autoimmune, metabolic, toxic, hereditary, and idiopathic pancreatitis constitute to the remaining number of the patient, and their symptoms typically recur despite medications or lifestyle modification. Abdominal pain is a leading cause of hospitalization in patients with chronic pancreatitis. Data from North American Pancreatitis Study 2 Continuation and Validation, a prospective multi-center study, showed that 66.8% of the patients experienced severe abdominal pain [8]. Such pain is commonly a result of pancreatic ductal obstruction secondary to stricture and stone formation although repetitive parenchymal inflammation also plays a major role in some non-obstructive cases. Medical treatments such as opioid-based analgesics or drugs that modulate neuropathic pain are effective for short term pain suppression, while more lasting pain control requires adequate pancreatic ductal drainage, which is chiefly done by endoscopic or surgical approach.
Stepwise escalation of treatment aggressiveness has been advocated [9][10], starting from oral analgesic regimens [11], followed by less invasive endoscopic drainage with or without extracorporeal shockwave lithotripsy for painful obstructive chronic pancreatitis. If these measures are deemed unsuccessful, surgery will be contemplated as the last resort [12][13]. The upside of this approach is that major surgery is avoided when the endoscopic treatment succeeds. A large multi-center retrospective study reported that endoscopic treatment resulted in long-term pain improvement in 80% of the patients [14]. However, a couple of studies reported that a significant proportion of patients remained in significant pain after a period of endoscopic treatment [15][16] and eventually needed a surgical procedure. In the literature, there are retrospective studies [17][18][19][20] and prospective randomized controlled trials (RCTs) [21][22][23] carried out to investigate the efficacy of different approaches to pain control in chronic pancreatitis. Moreover, recent RCT showed early surgery had lower pain scores compared with endoscopy-first approach [21].
2. Pain Relief after Endoscopic and Surgical Treatment
As to pain relief assessment, three studies [19][21][22] used the Izbicki pain score [24], one [23] used Melzack score [25], one [17] used reduction in dosage, and two [18][20] did not report their methods of pain relief assessment. Three studies [17][18][22] found no difference in pain relief between the two modalities, while four studies [19][20][21][23] reported superior pain relief with the surgical approach. Our meta-analysis of these seven studies demonstrated that surgical drainage was associated with better overall pain relief (complete and partial) as the primary outcome [OR 0.33, 95% CI 0.23–0.47, p < 0.001, I2 = 4%] (Figure 1). Four studies [18][21][22][23] reported both complete pain relief (Figure 2) and partial pain relief (Figure 3) as the secondary outcome. Although statistical difference was not demonstrable between the two treatment approaches regarding complete [OR 0.57, 95% CI 0.32–1.01, p = 0.054, I2 = 0%] and partial [OR 0.67, 95% CI 0.37–1.22, p = 0.19, I2 = 0%] pain relief, it was noted that surgical drainage tends to have a higher rate of complete pain relief (Figure 2).
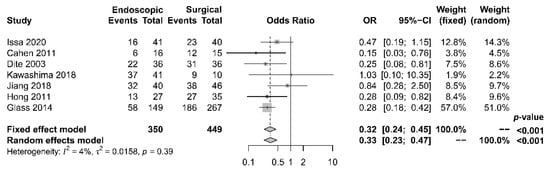
Figure 1. Forrest plot of the effect of endoscopy and surgery on overall (complete and partial) pain relief.
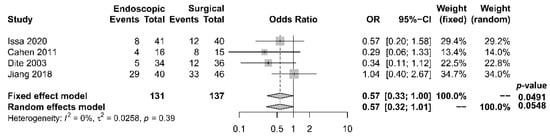
Figure 2. Forrest plot of the effect of endoscopy and surgery on complete pain relief.
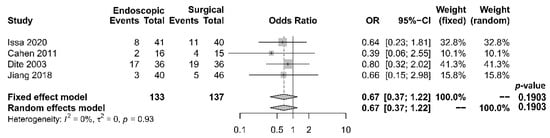
Figure 3. Forrest plot of the effect of endoscopy and surgery on partial pain relief.
3. Other Treatment Outcomes
3.1. Hospital Stay, Procedure-Related Complications, and Mortality
The length of hospital stay was reported in five studies [17][18][19][21][22], with a tendency of shorter stay in the endoscopic group. The median period of stay was 28.4 days in the endoscopic group and 36.8 days in the surgical group. Four [17][19][21][22] out of the five studies reported shorter stay in the endoscopic group, while one [18] reported that the total mean stay was longer in the endoscopic group, which had more hospital admissions. The single mean hospital stay was significantly shorter in the endoscopic group. Our meta-analysis, however, found no significant difference in length of hospital stay between the two groups [OR −0.54, 95% CI −1.23–0.15, p = 0.13, I2 = 87%] (Figure 4).
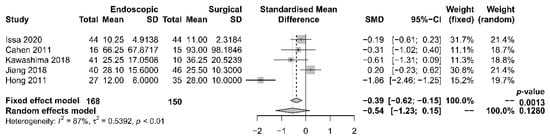
Figure 4. Forrest plot of the effect of endoscopy and surgery on length of hospital stay.
Rates of complication and mortality were reported in five studies [17][18][19][21][22]. The procedural mortality rate was 1.2% in the endoscopic group and 0.6% in the surgical group. No statistically significant difference in the occurrence of overall complication between the two groups was observed [OR 1.00, 95% CI 0.41–2.46, p = 0.99, I2 = 49%] (Figure 5).
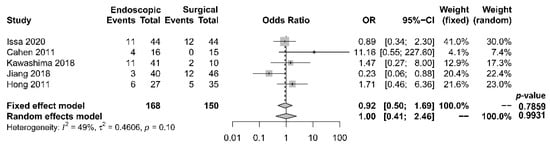
Figure 5. Forrest plot of the effect of endoscopy and surgery on complication rate.
3.2. Endocrine and Exocrine Insufficiency
Five papers [17][18][19][21][22] provided comparative data regarding endocrine insufficiency. It was noted that different definitions of endocrine insufficiency were adopted. Four studies [18][19][21][22] defined it as a new onset of diabetes mellitus or the need for glycemic control, whereas the other study [17] used the increase in HbA1c level > 6.1% as the definition. Despite the heterogeneity in the definitions adopted, all these studies reported superior outcomes with the surgical approach. The overall incidence of endocrine insufficiency was 29.8% in the endoscopic drainage group versus 20.0% in the surgical drainage group. The difference was statistically significant [OR 2.10, 95% CI 1.20–3.67, p = 0.01, I2 = 0%] (Figure 6).
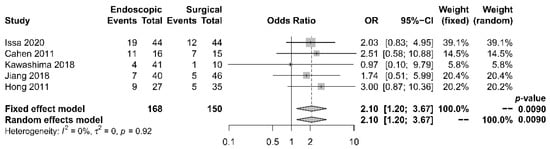
Figure 6. Forrest plot of the effect of endoscopy and surgery on endocrine insufficiency.
Six papers [17][18][19][20][21][22] reported data regarding exocrine insufficiency; similarly, different definitions were adopted. Exocrine insufficiency was defined as fecal elastase <200 μg/g in two studies [21][22], as new onset of steatorrhea in another [18], and as pancreatic functioning diagnosant level <70% in yet another [17]. The other two studies [19][20] did not report the assessment method for exocrine function. Meta-analysis was not possible due to the gross inconsistency of definitions. Nonetheless, these papers demonstrated slightly superior outcomes in the surgical group (Table 1). The overall incidence of exocrine insufficiency was 54.5% after endoscopy and 44.5% after surgery. The difference was, however, not statistically significant [p = 0.46].
4. Qualitative Assessment of the Included Studies
The three RCTs [21][22][23] had a mean Jadad score [26] of 2.67 (range 2–3), indicating medium quality (Table 1). Their methods of randomization were suitable and clearly defined. Two [21][22] out of the three papers reported withdrawal and dropout rates. However, treatment involving endoscopy and surgery made blinding impossible, which limited the quality of the three studies.
Table 1. Quality assessment for the included studies.
| Study | Design | Method of Grading | Jadad Scale | Newcastle–Ottawa Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomization | Blinding | Withdrawal and Dropout | Method of Randomization | Method of Blinding | Total | Selection | Comparability | Outcome | Total | |||
| Issa et al. (2020) | RCT | Jadad Scale | 1 | 0 | 1 | 1 | 0 | 3 | - | - | - | - |
| Cahen et al. (2011) | RCT | Jadad Scale | 1 | 0 | 1 | 1 | 0 | 3 | - | - | - | - |
| Dite et al. (2003) | RCT | Jadad Scale | 1 | 0 | 0 | 1 | 0 | 2 | - | - | - | - |
| Kawashima et al. (2018) | Retrospective, comparative cohort study | Newcastle–Ottawa Score | - | - | - | - | - | - | 2 | 2 | 2 | 6 |
| Jiang et al. (2018) | Retrospective, comparative cohort study | Newcastle–Ottawa Score | - | - | - | - | - | - | 4 | 2 | 3 | 9 |
| Hong et al. (2011) | Retrospective, comparative cohort study | Newcastle–Ottawa Score | - | - | - | - | - | - | 4 | 2 | 2 | 8 |
| Glass et al. (2014) | Retrospective, comparative cohort study | Newcastle–Ottawa Score | - | - | - | - | - | - | 3 | 1 | 2 | 6 |
The four retrospective cohort studies [17][18][19][20] had a mean NOS of 7.25 (range 6–9) (Table 2). The overall quality of the studies was satisfactory. Patient selection and treatment outcomes were clearly documented. Nonetheless, the study populations were not fully comparable due to treatment preferences concerning patients’ clinical situations. A brief follow-up period and a high dropout rate also limited the quality of a couple of the studies [17][23].
5. Assessment of Publication Bias of the Included Studies
Regarding the effect of drainage approach on pain relief (partial or complete), results from four studies [19][20][22][23] favored surgical drainage while those from three studies [17][18][21] favored endoscopic drainage. The funnel plot demonstrated an even distribution of the seven studies, suggesting insignificant publication bias [Eggar’s test p = 0.40] (Figure 7). Sensitivity analysis was performed to test the validity of I-square value in the pooled result (random effect model) of primary outcome. The optimal specificity and sensitivity (in the Youden index sense) for summary ROC curve are 0.8 and 0.523, respectively, resulting in a value of 0.677 for the area under the summary ROC curve, signifying consistent heterogeneity with the I-square test value (Figure 8).
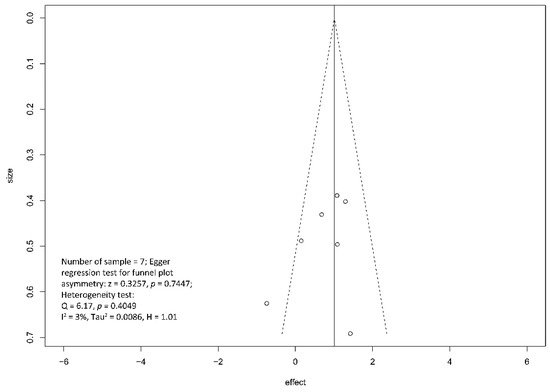
Figure 7. Funnel plot for the assessment of the presence of publication bias for pain relief (complete and partial) meta-analysis.
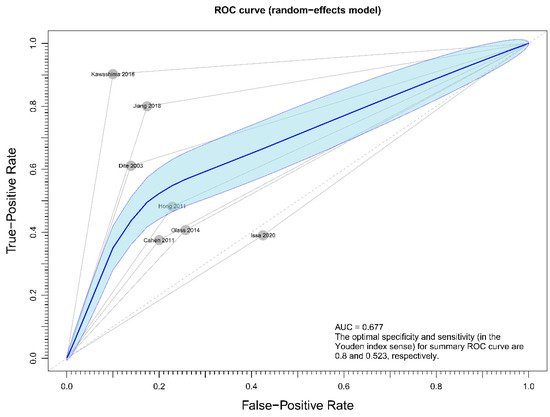
Figure 8. Figure showing sensitivity analysis on primary outcome (overall pain relief).
References
- Yadav, D.; Timmons, L.; Benson, J.T.; Dierkhising, R.A.; Chari, S.T. Incidence, prevalence, and survival of chronic pancreatitis: A population-based study. Am. J. Gastroenterol. 2011, 106, 2192–2199.
- Hirota, M.; Shimosegawa, T.; Masamune, A.; Kikuta, K.; Kume, K.; Hamada, S.; Kihara, Y.; Satoh, A.; Kimura, K.; Tsuji, I.; et al. The sixth nationwide epidemiological survey of chronic pancreatitis in Japan. Pancreatology 2012, 12, 79–84.
- Tandon, R.K.; Sato, N.; Garg, P.K. Chronic pancreatitis: Asia-Pacific consensus report. J. Gastroenterol. Hepatol. 2002, 17, 508–518.
- Wang, L.W.; Li, Z.S.; De Li, S.; Jin, Z.D.; Zou, D.W.; Chen, F. Prevalence and clinical features of chronic pancreatitis in China: A retrospective multicenter analysis over 10 years. Pancreas 2009, 38, 248–254.
- Coté, G.A.; Yadav, D.; Slivka, A.; Hawes, R.H.; Anderson, M.A.; Burton, F.R.; Brand, R.E.; Banks, P.A.; Lewis, M.D.; Disario, J.A.; et al. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin. Gastroenterol. Hepatol. 2011, 9, 266–273.
- Frulloni, L.; Gabbrielli, A.; Pezzilli, R.; Zerbi, A.; Cavestro, G.M.; Marotta, F.; Falconi, M.; Gaia, E.; Uomo, G.; Maringhini, A.; et al. Chronic pancreatitis: Report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig. Liver Dis. 2009, 41, 311–317.
- Hall, T.C.; Garcea, G.; Webb, M.A.; Al-Leswas, D.; Metcalfe, M.S.; Dennison, A.R. The socio-economic impact of chronic pancrea-titis: A systematic review. J. Eval. Clin. Pract. 2014, 20, 203–207.
- Wilcox, C.M.; Yadav, D.; Ye, T.; Gardner, T.B.; Gelrud, A.; Sandhu, B.S.; Lewis, M.; Al-Kaade, S.; Cote, G.A.; Forsmark, C.E.; et al. Chronic Pancreatitis Pain Pattern and Severity Are Independent of Abdominal Imaging Findings. Clin. Gastroenterol. Hepatol. 2015, 13, 552–560.
- American Gastroenterological Association Medical Position Statement: Treatment of pain in chronic pancreatitis. Gastroenterology 1998, 115, 763–764.
- Dumonceau, J.-M.; Kapral, C.; Aabakken, L.; Papanikolaou, I.S.; Tringali, A.; Vanbiervliet, G.; Beyna, T.; Dinis-Ribeiro, M.; Hritz, I.; Mariani, A.; et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2020, 52, 127–149.
- Jadad, A.R.; Browman, G.P. The WHO analgesic ladder for cancer pain management: Stepping up the quality of its evaluation. JAMA 1995, 274, 1870–1873.
- Forsmark, C.E. Management of Chronic Pancreatitis. Gastroenterology 2013, 144, 1282–1291.e3.
- Drewes, A.M.; Bouwense, S.A.W.; Campbell, C.M.; Ceyhan, G.O.; Delhaye, M.; Demir, I.E.; Garg, P.K.; Van Goor, H.; Halloran, C.; Isaji, S.; et al. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology 2017, 17, 720–731.
- Rösch, T.; Daniel, S.; Scholz, M.; Huibregtse, K.; Smits, M.; Schneider, T.; Ell, C.; Haber, G.; Riemann, J.-F.; Jakobs, R.; et al. Endoscopic Treatment of Chronic Pancreatitis: A Multicenter Study of 1000 Patients with Long-Term Follow-Up. Endoscopy 2002, 34, 765–771.
- Lankisch, P.G. Natural Course of Chronic Pancreatitis. Pancreatology 2001, 1, 3–14.
- Ammann, R.W.; Muellhaupt, B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology 1999, 116, 1132–1140.
- Kawashima, Y.; Kawaguchi, Y.; Kawanishi, A.; Ogawa, M.; Hirabayashi, K.; Nakagohri, T.; Mine, T. Comparison between Endoscopic Treatment and Surgical Drainage of the Pancreatic Duct in Chronic Pancreatitis. Tokai J. Exp. Clin. Med. 2018, 43, 117–121.
- Jiang, L.; Ning, D.; Cheng, Q.; Chen, X.-P. Endoscopic versus surgical drainage treatment of calcific chronic pancreatitis. Int. J. Surg. 2018, 54, 242–247.
- Hong, J.; Wang, J.; Keleman, A.M.; Imagawa, D.K.; Xu, K.; Wang, W.; Liu, E.; Niu, W.; Wang, J.; Sun, Q.; et al. Endoscopic versus surgical treatment of downstream pancreatic duct stones in chronic pancreatitis. Am. Surg. 2011, 77, 1531–1538.
- Glass, L.M.; Whitcomb, D.C.; Yadav, D.; Romagnuolo, J.; Kennard, E.; Slivka, A.A.; Brand, R.E.; Anderson, M.A.; Banks, P.A.; Lewis, M.; et al. Spectrum of Use and Effectiveness of Endoscopic and Surgical Therapies for Chronic Pancreatitis in the United States. Pancreas 2014, 43, 539–543.
- Issa, Y.; Kempeneers, M.A.; Bruno, M.J.; Fockens, P.; Poley, J.W.; Ali, U.A.; Bollen, T.L.; Busch, O.R.; Dejong, C.H.; van Duijvendijk, P.; et al. Effect of early surgery vs endoscopy-first approach on pain in patients with chronic pancreatitis: The ESCAPE randomized clinical trial. JAMA 2020, 323, 237–247.
- Cahen, D.L.; Gouma, D.J.; Laramée, P.; Nio, Y.; Rauws, E.A.; Boermeester, M.A.; Busch, O.R.; Fockens, P.; Kuipers, E.J.; Pereira, S.P.; et al. Long-term outcomes of endoscopic vs surgical drainage of the pancreatic duct in patients with chronic pancreatitis. Gastroenterology 2011, 141, 1690–1695.
- Díte, P.; Ružicka, M.; Zboril, V.; Novotný, I. A Prospective, Randomized Trial Comparing Endoscopic and Surgical Therapy for Chronic Pancreatitis. Endoscopy 2003, 35, 553–558.
- Izbicki, J.R.; Bloechle, C.; Broering, D.C.; Kuechler, T.; Broelsch, C.E. Longitudinal V-Shaped Excision of the Ventral Pancreas for Small Duct Disease in Severe Chronic Pancreatitis. Ann. Surg. 1998, 227, 213–219.
- Melzack, R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain 1975, 1, 277–299.
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12.
More
Information
Subjects:
Pathology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
562
Revisions:
2 times
(View History)
Update Date:
16 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




