Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhou Fang | + 105 word(s) | 105 | 2022-02-21 10:04:17 | | | |
| 2 | Beatrix Zheng | + 2447 word(s) | 2552 | 2022-02-22 03:34:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fang, Z. Research Status of Graphene Polyurethane Composite Coating. Encyclopedia. Available online: https://encyclopedia.pub/entry/19708 (accessed on 08 February 2026).
Fang Z. Research Status of Graphene Polyurethane Composite Coating. Encyclopedia. Available at: https://encyclopedia.pub/entry/19708. Accessed February 08, 2026.
Fang, Zhou. "Research Status of Graphene Polyurethane Composite Coating" Encyclopedia, https://encyclopedia.pub/entry/19708 (accessed February 08, 2026).
Fang, Z. (2022, February 21). Research Status of Graphene Polyurethane Composite Coating. In Encyclopedia. https://encyclopedia.pub/entry/19708
Fang, Zhou. "Research Status of Graphene Polyurethane Composite Coating." Encyclopedia. Web. 21 February, 2022.
Copy Citation
Graphene material has a variety of excellent properties and applications in energy storage, biomaterials, photoelectric devices, and other fields. With the progress of nanotechnology, graphene nanomaterials have shown their advantages in the field of new nano-corrosion coatings with their high barrier structure. In addition, polyurethane is also widely used in the field of anti-corrosion coatings due to its excellent chemical resistance, mechanical properties, and weathering resistance. The preparation of composite coatings by combining graphene nanomaterials with traditional polyurethane (PU) coatings has opened up a new way for the research and development of new anticorrotic coatings.
graphene oxide
polyurethane
composite coating
corrosion protection
1. Graphene Material
Graphene is a carbon heterostructure. It is a monolayer of flat plates of carbon atoms with sp2 hybridization orbitals, densely packed in a honeycomb structure [1]. Its structure is shown in Figure 1. The graphene material has ultra-high strength with a Young’s modulus of about 1 TPa [2]. It also has a high thermal conductivity of about 5300 W/m/K [3] and an ultra-high surface area of 2630 m2/g [4]. Graphene materials have many excellent properties such as chemical inertness [5], thermal stability [6], gas impermeability [7], and toughness. Researchers have used these properties to try to use graphene materials for various applications. Among them, scholars in the field of protective coatings have started to experiment with graphene as part of protective coating systems and have initially demonstrated that graphene and its derivatives can improve the physical properties of polymeric nanocomposites [8][9]. Graphene nanocomposite coatings have great potential for application in material corrosion protection and will lead to the development of new coatings.
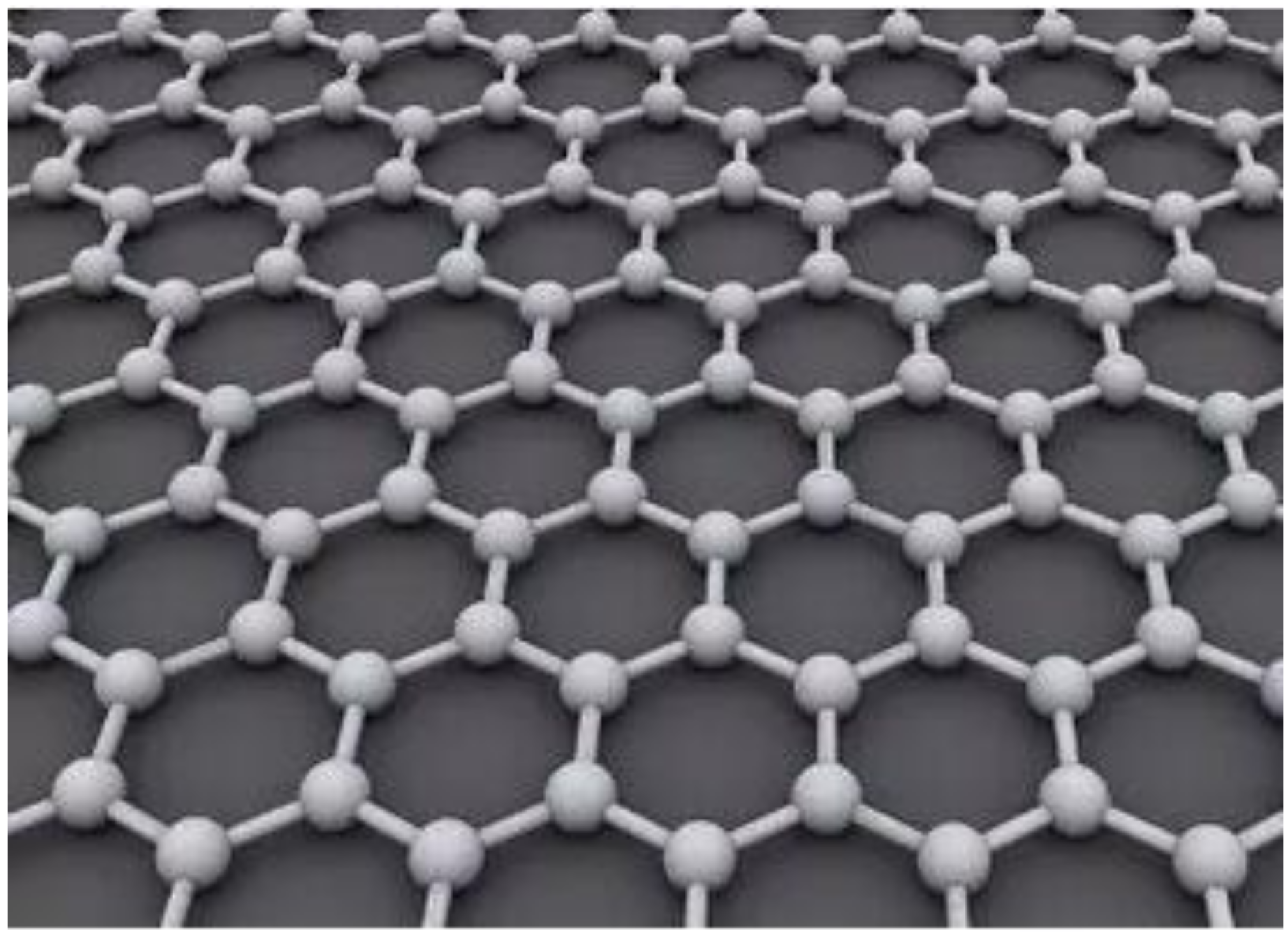 Figure 1. Schematic diagram of graphene structure.
Figure 1. Schematic diagram of graphene structure.At present, reduced graphene (G), reduced graphene oxide (RGO), GO, functional group-modified graphene, and composites based on the above graphene are often applied in organic anti-corrosive coatings. GO is an oxide of graphene. Compared with pure graphene, GO has more oxygen-containing functional groups. In addition, GO has many properties such as being more active than graphene, easy to chemically modify, low production cost, and can be mass produced. GO contains four main oxygen-containing functional groups: hydroxyl, epoxide, carbonyl, and carboxyl groups. In addition, it contains small amounts of esters, ethers, and phenols [10]. The hydroxyl and epoxy groups are mainly distributed on the sheet of GO, while the carbonyl and carboxyl groups are mainly located at the edges of GO [11]. Its molecular structure is shown in Figure 2. GO can remain stable in aqueous solutions and polar solvents due to the presence of a large number of oxygen-containing groups. Compared with graphene, GO has higher chemical stability and can be used as a precursor and support carrier for the synthesis of graphene composites. It is characterized by easy functionalization and high controllability.
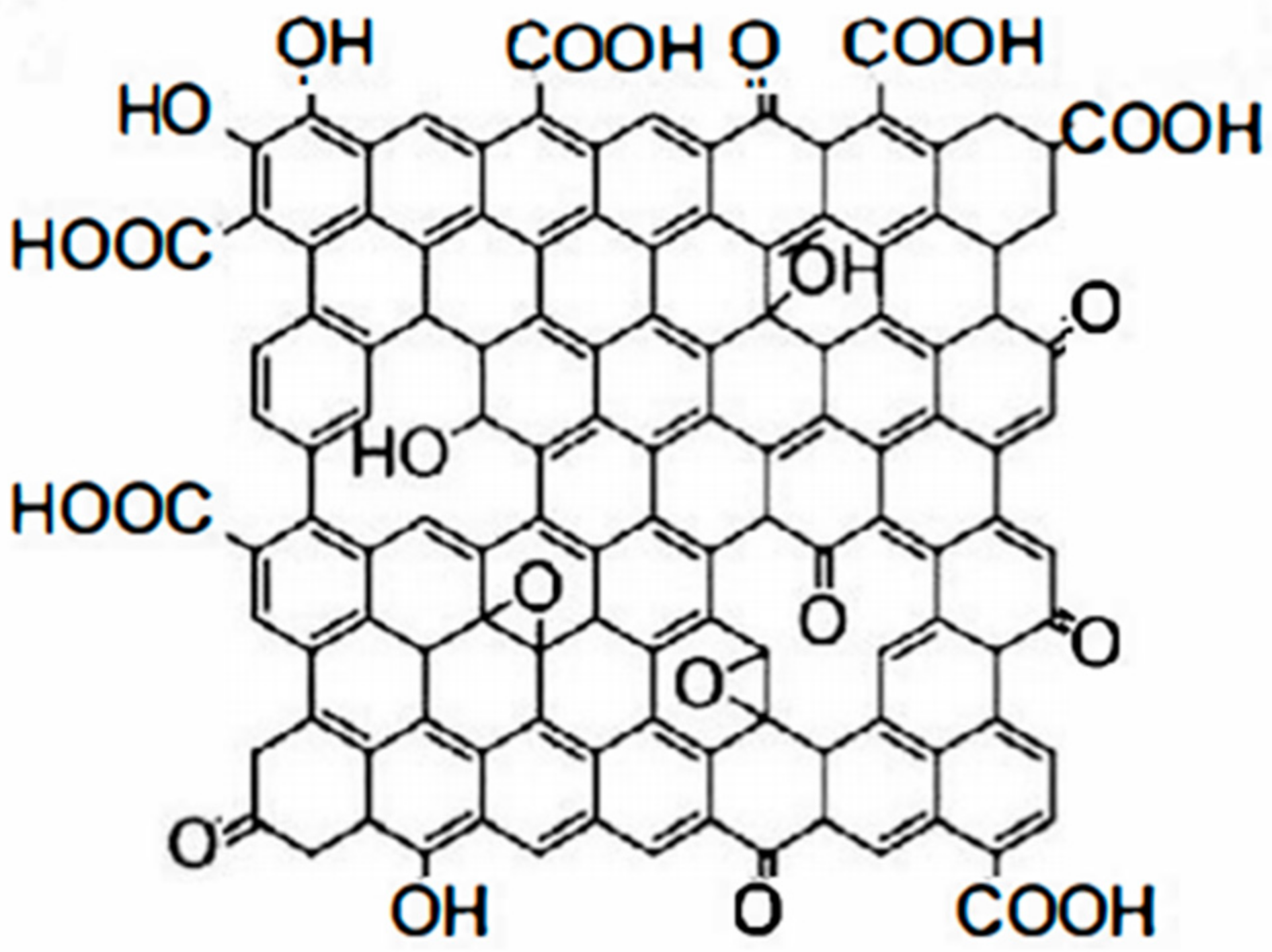 Figure 2. Molecular structure of GO.
Figure 2. Molecular structure of GO.The molecular structure differences between GO and graphene are a double-edged sword, with both advantages and disadvantages. Graphene consists almost entirely of sp2 hybridized carbon atoms, but GO contains not only sp2 hybridized regions, but also a large number of sp3 hybridized regions. Structurally, GO has oxygen-containing groups on its edges and surfaces, but it also brings certain defects. It is completely different from graphene in terms of mechanical properties, electrical properties, thermal conductivity, and surface properties. The excellent properties of graphene are due to its highly conjugated structure. However, the oxygen-containing functional groups and damaged pores in GO severely disrupt this highly conjugated structure, making GO less capable than graphene [12][13].
As an important oxygen-containing derivative of graphene, GO has many lattice defects in its structure, leading to degradation of its electrical conductivity, thermal conductivity, and other properties. Therefore, it is difficult to apply it in the preparation of nanocomposites that have strict requirements for its structural integrity. However, GO still has great advantages in the preparation of nanocomposites with excellent mechanical properties, wear resistance, adsorption properties, etc. The presence of a large number of oxygen-containing groups gives GO a significant advantage in terms of water dispersibility, amphiphilicity, degree of modifiability, and compatibility with a polymer matrix.
RGO is obtained by treating GO using a series of methods such as reductant reduction, thermal reduction, and electrochemical reduction. In this process, the structural damage caused by oxidation is partially repaired during the reduction of GO, making the reduced GO stronger than GO. The process of reducing GO is actually a process of repairing the conjugated structure of graphene to restore the properties of GO [14]. Therefore, in order to overcome the structural defects of GO, researchers usually use GO as a precursor material in the preparation of graphene-based composites and prepare RGO by chemical reduction. Then, the composites are prepared by mixing with other substrates. The reduction process of GO is shown in Figure 3. The RGO prepared by GO reduction retains various rich oxygen-containing functional groups at the edges and surfaces, which makes it easier to be chemically modified than pure graphene materials, thus improving its compatibility with other substrates.
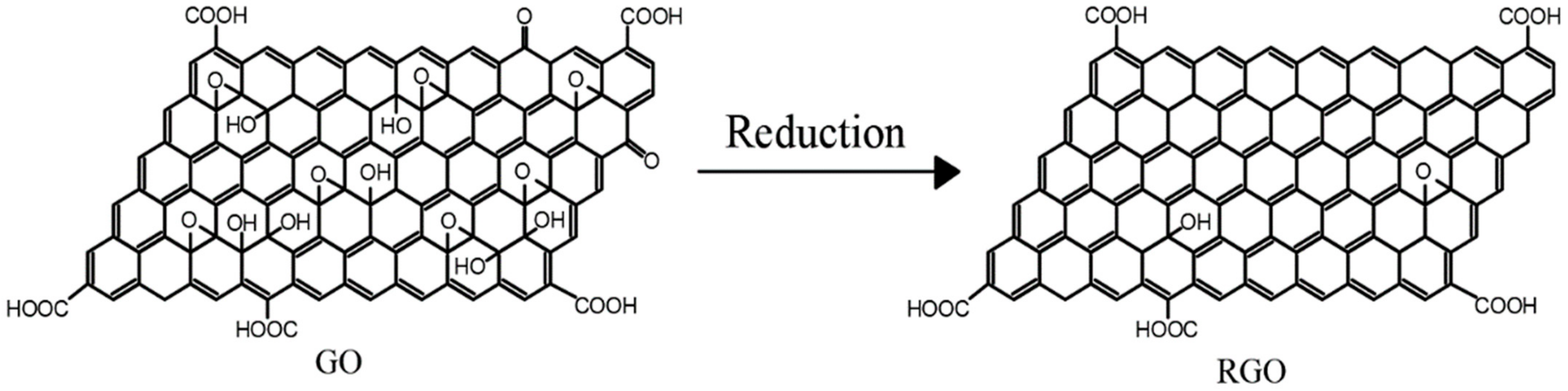 Figure 3. Reduction of GO.
Figure 3. Reduction of GO.2. Coating Preparation Processes
2.1. Laser Cladding Technology
Laser cladding technology is the simultaneous, instantaneous melting and rapid solidification of the required alloy powder with the very thin surface of the substrate, forming a dense metallurgical bonded clad alloy layer on the substrate. Laser cladding offers a wide choice of cladding materials due to the fine organization of the cladding layer, which can be used to achieve high corrosion resistance, high wear resistance, high hardness, and other performance requirements. The main production methods of laser cladding are divided into the pre-set cladding method and the simultaneous powder feeding method [15]. The pre-setting method has a long process flow, complex procedures, poor coating uniformity, high laser power requirements, and the decomposition of the bonding agent can easily cause contamination of the clad layer, which may form defects such as porosity and cracking. The advantage of synchronous powder feeding is that the process is relatively simple and can be controlled automatically with high efficiency, which has been popularized and applied in many enterprises. Currently, ultra-high speed laser cladding technology is very popular. Compared with ordinary laser cladding technology, ultra-high speed laser cladding technology forms finer tissues; however, there are defects caused by unmelted particles and uneven heat distribution of the spot on the surface of the clad layer during the cladding process. To improve the surface quality of the cladding layer, post-treatment is required. Bai et al. [16] prepared iron-based coatings by high-speed laser melting and strongly spun the outer surface of the coatings, and the results showed that the strongly spun coatings exhibited higher corrosion resistance than the untreated coatings. Zhang et al. [17] prepared graphene-reinforced Ti6Al4V composite coatings with good bonding of graphene to the Ti6Al4V substrate.
2.2. Electron Beam Melting Technology
Electron beam cladding technology uses high energy electron beam into the material; the energy is instantly deposited on the surface of the coating, generating heat to make the coating material melt, the substrate partially melts, and the substrate and coating combine to form a coating, improving the surface properties of the material. Electron beam coating is applied to the surface of the coating material by thermal spraying, binder, press, and other processing methods; pre-coating a layer of alloy powder coating with thickness ranging from a few microns to a few millimeters and with special physicochemical properties; and then using high energy density electron beam to irradiate and scan the surface of the coating material. The treated coating instantly melts and fuses with the substrate to form a new alloy layer, thus obtaining the structure and properties compatible with the design requirements. The structure and properties of the coating are compatible with the design requirements, i.e., a coating with wear resistance, corrosion resistance, and high temperature oxidation resistance [18]. Wang et al. [19] prepared single-, double-, and triple-layer WC-40Co coatings by electron beam cladding and analyzed the wear resistance and corrosion resistance of the coatings, which showed that the wear resistance of the coatings increased with the number of cladding layers and could reach 11.5 times the wear resistance of the substrate. The corrosion resistance of the single- and double-layer coatings was better than that of the substrate, however, the surface of the triple-layer coating was significantly damaged by cracks.
2.3. Thermal Spraying Technology
Thermal spraying technology includes flame spraying, plasma spraying [20], laser spraying [21], etc. The use of a heat source to atomize the material with the help of high-speed gas will be sprayed on the surface of the substrate and quickly cooled and deposited into a certain function of the coating technology. Thermal spraying technology can generally prepare anti-oxidation, corrosion resistance, wear resistance, and other coatings. Thermal spraying technology has been widely used in aerospace, marine field, automotive field, petrochemical field, etc. The coating materials for thermal spraying can be chosen from metals, alloys, ceramics, and composites. Thermal spraying technology can be used for a wide range of substrates and coatings, with no restrictions on size and shape and little deformation of the workpiece. In the spraying process, the influence on the performance of the substrate is small, the coating thickness control range is large, and the coating thickness usually reaches 100–400 μm, but in the spraying of smaller areas of the parts, the economy is poor. When preparing coatings by thermal spraying, the cleanliness and roughness of the substrate surface affects the bonding properties, so the substrate is usually subjected to surface cleaning and roughening processes before spraying. Muzika Lukáš et al. [22] prepared metal coatings using three different spraying methods: twin-wire arc spraying (TWAS), flame spraying (FS), and high-speed oxygen fuel spraying (HVOF). Forati Tahmineh et al. [23] used atmospheric plasma spraying and high speed oxygen fuel as a Cu-graphene nanosheet composite coating to develop a multifunctional and scalable surface engineering technique. The results showed that the corrosion resistance of Cu with the coating was improved by 89% compared to the Cu surface without the coating.
2.4. Brazing Technology
Brazing is a material joining method that enables a tight metallurgical bond to be formed between the base material and the additive material. Compared with other welding methods, brazing technology is more suitable for the preparation of coatings on single crystal high temperature alloys. The most important feature of brazing is that the welding temperature is low and the base material does not melt, reducing the possibility of recrystallization and warming; the brazing process uses the wetting and solidifying effect of the molten brazing material to connect with the base material, so the strength is higher than that of spray coatings [24]. However, the diffusion and reaction of C and Si elements at the interface during the brazing process can destabilize the structure. Wang et al. [25] improved the performance of vacuum brazed NiCr-Cr3C2 coatings on single crystal high temperature alloys by adjusting the Cr content to control the amount of Si or C diffusion into the base material.
2.5. Vapor Deposition Technology
Vapor deposition technology is divided into physical vapor deposition (PVD) and chemical vapor deposition (CVD), and as a new green coating technology, PVD is widely used in mechanical, electronic, and optical industries because of its high hardness, good wear resistance, low friction coefficient, stable chemical properties, and heat and oxidation resistance. To protect the substrate against corrosion by PVD technology, the coating must be well shielded and isolated from the corrosive medium and the substrate material, and the coating itself must be stable in the corrosive medium and maintain a low corrosion rate. The corrosion resistance of coatings can be improved by selecting the appropriate chemical composition, reducing the growth and preparation defects to obtain a dense and uniform coating structure, increasing the coating thickness, using a multilayer film structure, pre-treating the coating, and adding a transition layer [26]. Jiang et al. [27] laminated pure Mg films onto hot-dip coated Zn-55Al-1.6Si steel by PVD, varying the Ar gas pressure and deposition. It was found that the lamination of Mg film enhanced the corrosion resistance, which was significantly affected by the thickness of Mg film, especially for the cross section by a factor of 2–10.
Chemical vapor deposition (CVD) is a technique in which various gases are introduced into a reaction chamber where a chemical reaction occurs on the surface of a substrate and the resulting solid products are deposited on the surface to form a thin film. These gases include gaseous reactants or vapors of liquid reactants that can form thin film elements, as well as other reaction gases. In order to obtain specific thin films by CVD, it is necessary to select the appropriate reaction method and to determine parameters such as temperature, gas composition, concentration, and pressure [28]. Nadeem Aamir et al. [29] deposited boron nitride-based coatings on mild steel substrates by chemical reaction of boron powder and ammonia gas in a CVD apparatus at 1200 °C. It was shown that the salt resistance of the boron nitride nanosheet coating was six times higher than that of bare mild steel. Samira Naghdi et al. [30] performed chemical vapor deposition of graphene on molybdenum and platinum at atmospheric pressure and showed that graphene exhibited good protective properties and could be used as a corrosion barrier.
2.6. Surface Composite Ion Treatment Technology
Surface composite ion treatment is a surface treatment process that combines two or more surface technologies to prepare composite coatings, film layers, and composite modified coatings. It includes ion implantation compounded with coating technology, laser or electron beam compounded with vapor deposition technology, and plasma spray compounded with laser technology. In ion-beam-assisted deposition processes, ion bombardment can increase the density of the film, eliminate or reduce the inherent stresses in the film layer, and improve the properties of the film layer, and a wide transition zone between the film atoms and the substrate atoms can be obtained by ion bombardment, which is extremely beneficial for improving the film/substrate bond. Xu et al. [31] deposited in situ graphene/silicon carbide composite coatings with <111> orientation by laser chemical vapor deposition using hexamethylsilane as a precursor. The study of the growth mechanism showed that the photolytic effect of the laser played an important role in the deposition process.
The coating preparation process is not only related to the characteristics of the coating itself, but also influenced by a variety of factors, such as the type of substrate, the performance of the coating, the type of application environment, and the functional requirements of the coating. With the advancement of science and technology, the coating preparation process and technology are developing rapidly in the direction of intelligence, efficiency and advancement. All the preparation processes introduced above can prepare anti-corrosion coatings, and at present, thermal spray technology, laser cladding technology, and vapor deposition technology are more widely used.
3. Conclusions and Prospect
Graphene materials have great application potential in the coating field. GO, as a nano-filler, can effectively improve the mechanical properties and anti-corrosion properties of PU coatings. Due to the large number of oxygen-containing functional groups on the surface of GO, it can be directly added to the PU matrix as a nano-filler. However, the performance of the composite will be reduced because of their strong interaction. Therefore, modification of GO is essential. Modified GO can react better with the PU matrix and have better dispersion in the PU matrix, so the performance of the composite material will be significantly improved. Considerable progress and development has been made in the application of GO in PU coatings, but there is still a lot of research that needs to be done on the application of GO.
References
- Eigler, S. Graphene. An introduction to the fundamentals and industrial applications edited by Madhuri Sharon and Maheshwar Sharon. Angew. Chem. Int. Ed. 2016, 55, 5122.
- Bai, S.; Shen, X. Graphene–inorganic nanocomposites. RSC Adv. 2012, 2, 64–98.
- Yoo, B.M.; Shin, H.J.; Yoon, H.W.; Park, H.B. Graphene and graphene oxide and their uses in barrier polymers. J. Appl. Polym. Sci. 2013, 131.
- Wang, Y.; Wang, X.; Guo, Z.; Chen, Y. Ultrafast coating procedure for graphene on solid-phase microextraction fibers. Talanta 2013, 119, 517–523.
- Ray, S.C. Application and Uses of Graphene; Elsevier: Amsterdam, The Netherlands, 2015.
- Lee, K.E.; Oh, J.J.; Yun, T.; Kim, S.O. Liquid crystallinity driven highly aligned largegraphene oxide composites. Solid State Chem. 2015, 224, 115–119.
- Cui, Y.; Kundalwal, S.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2015, 98, 313–333.
- Monetta, T.; Acquesta, A.; Bellucci, F. Graphene/epoxy coating as multifunctional material for aircraft structures. Aerospace 2015, 2, 423–434.
- Qi, K.; Sun, Y.; Duan, H.; Guo, X. A corrosion-protective coating based on a solution-processable polymer-grafted graphene oxide nanocomposite. Corros. Sci. 2015, 98, 500–506.
- Voiry, D.; Yang, J.; Kupferberg, J.; Fullon, R.; Lee, C.; Jeong, H.Y.; Shin, H.S.; Chhowalla, M. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 2016, 353, 1413–1416.
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482.
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924.
- Mahanta, N.K.; Abramson, A.R. Thermal conductivity of graphene and graphene oxide nanoplatelets. In Proceedings of the 13th InterSociety Conference on Thermal and Thermomech anical Phenomena in Electronic Systems, San Diego, CA, USA, 30 May–1 June 2012; pp. 1–6.
- Pei, S.F.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228.
- Xiao, L.L.; Ren, Y.; Gao, Q.H. Introduction to the research progress of laser cladding technology. New Technol. New Technol. 2021, 7, 5–7. (In Chinese)
- Bai, Q.F.; Zhang, J.; Li, Q.H. Microstructure and corrosion resistance of Fe-based coatings prepared using high-speed laser cladding and powerful spinning treatment. Mater. Lett. 2022, 310, 131429.
- Zhang, L.; Zhao, Z.; Bai, P.; Du, W.; Li, Y.; Yang, X.; Wang, Q. In-situ synthesis of TiC/graphene/Ti6Al4V composite coating by laser cladding. Mater. Lett. 2020, 270, 127711, (prepublish).
- Lu, R.X.; Liu, H.L.; Wang, X.Y. Research status and development of electron beam cladding technology. Therm. Process. Technol. 2022, 15–19. (In Chinese)
- Wang, W.Q.; Zhang, S.Q.; Xiao, S. Microstructure and properties of multilayer WC-40Co coating on Ti-6Al-4V by electron beam cladding. Mater. Charact. 2022, 183, 111585.
- Rao, M.S.; Ramesh, M.R.; Ravikiran, K. Developing partially oxidized NiCr coatings using the combined flame spray and plasma spray process for improved wear behaviour at high temperature. Wear 2021, 478, 203885.
- Chen, H.X.; Kong, D.J. Comparison on electrochemical corrosion performances of arc and laser thermal sprayed Al–Ti–Ni coatings in marine environment. Mater. Chem. Phys. 2020, 251, 123200.
- Lukáš, M.; Michal, Š.; Šárka, H.; Petra, Š. Application of flash-pulse thermography methods for quantitative thickness inspection of coatings made by different thermal spraying technologies. Surf. Coat. Technol. 2021, 406, 126748.
- Tahmineh, F.; Navid, S.; Tatiana, K.; Ben, E.F.; Nima, M.; Martin, P.; Ali, D.; Christian, M. Wetting and corrosion characteristics of thermally sprayed copper-graphene nanoplatelet coatings for enhanced dropwise condensation application. Carbon Trends 2021, 3, 100018.
- Wu, N.; Li, Y.; Ma, Q. Microstructure evolution and shear strength of vacuum brazed joint for super-Ni/NiCr laminated composite with Ni–Cr–Si–B amorphous interlayer. Mater. Des. 2014, 53, 816–821.
- Wang, D.; Wang, W.Q.; Chen, X.G.; Chi, C.T.; Wang, M.S.; Han, X.; Xie, Y.J. Influence of Cr addition on the interface purification of vacuum brazed NiCr-Cr3C2 coatings on single crystal superalloy. Surf. Coat. Technol. 2017, 325, 200–209.
- Gu, J.F.; Li, P.; Zhong, Q.D. Application of physical vapor deposition in corrosion resistant coatings. Mater. Guide 2016, 30, 75–80. (In Chinese)
- Jiang, Q.; Liao, X.; Yang, J.; Wang, G.; Chen, J.; Tian, C.; Li, G. A two-step process for the preparation of thermoplastic polyurethane/graphene aerogel composite foams with multi-stage networks for electromagnetic shielding. Compos. Commun. 2020, 21, 100416.
- Niu, Y. Research and application progress of chemical vapor deposition technology. Sci. Technol. Wind. 2020, 13, 161. (In Chinese)
- Aamir, N.; Faheem, M.M.; Ali, R.M.; Tasaduq, I.M.; Javaid, I.M.; Ur, R.Z. Binder free boron nitride-based coatings deposited on mild steel by chemical vapour deposition: Anti-corrosion performance analysis. Phys. B Condens. Matter 2020, 602, 412600, (prepublish).
- Naghdi, S.; Nešović, K.; Mišković-Stanković, V.; Rhee, K.Y. Comprehensive electrochemical study on corrosion performance of graphene coatings deposited by chemical vapour deposition at atmospheric pressure on platinum-coated molybdenum foil. Corros. Sci. 2018, 130, 31–44.
- Xu, Q.; Deng, Z.; Sun, Q.; Tu, R.; Zhang, S.; Yang, M.; Li, Q.; Zhang, L.; Goto, T.; Ohmori, H. Electrically conducting graphene/SiC(111) composite coatings by laser chemical vapor deposition. Carbon 2018, 139, 76–84.
More
Information
Subjects:
Biochemical Research Methods
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
22 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




