Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Brigida Anna Maiorano | + 2023 word(s) | 2023 | 2021-09-07 04:53:05 | | | |
| 2 | Bruce Ren | -13 word(s) | 2010 | 2021-09-13 04:26:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Maiorano, B.A. ICIs in Ovarian Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/14110 (accessed on 08 February 2026).
Maiorano BA. ICIs in Ovarian Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/14110. Accessed February 08, 2026.
Maiorano, Brigida Anna. "ICIs in Ovarian Cancer" Encyclopedia, https://encyclopedia.pub/entry/14110 (accessed February 08, 2026).
Maiorano, B.A. (2021, September 11). ICIs in Ovarian Cancer. In Encyclopedia. https://encyclopedia.pub/entry/14110
Maiorano, Brigida Anna. "ICIs in Ovarian Cancer." Encyclopedia. Web. 11 September, 2021.
Copy Citation
Ovarian cancer (OC) represents the fifth leading cause of cancer-related deaths among women. In the advanced disease setting, OC recurrence after chemotherapy is over 70% in the first 2 years, with few therapeutic options. Immunotherapy with the immune checkpoint inhibitors (ICIs) showed high efficacy and changed the therapeutic scenario of many tumors in the last 10 years.
ovarian cancer
checkpoint inhibitors
ICIs
immunotherapy
PARP
avelumab
pembrolizumab
nivolumab
bevacizumab
platinum
1. Introduction
Ovarian cancer (OC) accounts for about 2% of tumors, representing the eighth most common cancer among the female population. The incidence is around 11 cases/100,000 inhabitants/year, and it is higher among white women [1][2]. The frequency of OC rises with age, being uncommon before 30, and more frequently presenting at 50–70. Globally, ovarian cancer represents the fifth leading cause of female cancer-related deaths, with a 5 y survival rate falling from 90% at stage I to 25% at stage IV [2]. The majority of OCs have an epithelial origin, among whom serous carcinoma has the most aggressive features and is usually diagnosed at advanced stages [3]. Platinum-based chemotherapy regimens represent the mainstay of treatment [4][5][6][7]. The response to these agents and the treatment-free interval (TFI) after platinum define the subsequent treatment, moving from the platinum-refractory (PR) (relapse < 6 months from the platinum end) to the platinum-sensitive (PS) patients (TFI > 12 mos). Despite initial benefits, disease recurrence occurs in over 2/3 of patients within the first two years. Therefore, new drugs were explored, and other agents such as the PARP-inhibitor (PARPi) agents and the anti-vascular endothelial growth factor (VEGF) bevacizumab were approved in the advanced setting [8][9].
Immunotherapy has represented a breakthrough therapy for many solid tumors [10]. Thus far, the best-studied mechanisms for inducing an immune response against tumors rely on inhibiting the immune checkpoint. The immune checkpoint inhibitors (ICIs) consist of monoclonal antibodies targeting Programmed Cell Death Protein 1 (PD-1)/Programmed Death-Ligand 1 (PD-L1) or Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4), expressed by tumor or immune cells. After binding with these ligands, ICIs remove the inhibition signals for the immune system, unlocking the anti-tumor response [11]. However, in OC, ICIs reported modest results, and some phase III trials were prematurely terminated for futility. Combinations with other compounds, such as PARPis or anti-angiogenic drugs, represent promising opportunities to enhance the clinical effectiveness of immunotherapy [12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36].
2. ICIs in Ovarian Cancer
Given the impact on morbidity and mortality among the female population, the search for new therapeutic options represents an unmet need for OC. Immunotherapy has revolutionized the treatment landscape of many solid tumors in the last ten years, and it now represents the first therapeutic approach with impressive survival benefits in diseases such as lung cancer, melanoma, renal cell carcinoma [10]. However, limited benefits have emerged in OC, even leading to premature termination due to the futility of some studies. Different components of the OC tumor microenvironment (TME) contribute to this failure, such as myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), T-cells, cytokines, and soluble factors [37][38][39][40]. MDSCs exert immunosuppressive functions, such as the inhibition of T-effector and natural killer (NK)-cells, and are induced under pro-inflammatory cytokines, IFNγ, tumor necrosis factor-alpha (TNFα), interleukin (IL)-6 [41]. In OC, IL-6 plays a negative prognostic role and is associated with high MDSCs, and tumor progression [42][43]. The inflammatory cytokines cooperate to induce cyclooxygenase-2 (COX-2) and lead to prostaglandin E2 (PGE2) synthesis, which limits T-cell recruiting at tumor sites, together with VEGF [44][45]. TAMs are recruited at ovarian tumor sites, and IL-6, IL-10, transforming growth factor (TGF)-β promote their differentiation in M2 macrophages, associated with tumor invasiveness, spread, and angiogenesis [46][47][48]. M2 macrophages increase with the OC stage when contemporary M1 macrophages decrease, playing a negative prognostic role [49][50][51]. Moreover, they promote immunosuppression by producing cytokines (IL-1R, IL-10, C-C Motif Chemokine Ligand [CCL]17, CCL20, CCL22) that inhibit T-effectors proliferation and enhance Tregs function [52][53][54]. Treg cells are associated with advanced stages of OC and have a negative prognostic and immunosuppressive role [54]. They produce IL-10 and TGFβ, contributing to the inhibition of effector T-cells [55]. High levels of immunosuppressive elements within OC TME can also weaken dendritic cells and antigen-presenting cells (APCs) activity [56]. More accurate knowledge of the TME of the primary tumors and the metastatic sites will facilitate the design of more effective treatment combinations (Figure 1).
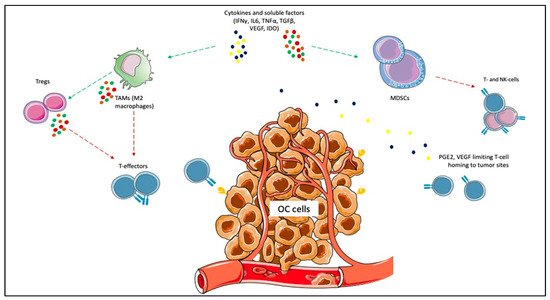
Figure 1. Immunosuppressive elements of ovarian cancer (OC) microenvironment. Cytokines and other soluble factors, such as interferon-gamma (IFNγ), tumor necrosis factor-alpha (TNFα), interleukin (IL)-6, IL-10, and transforming growth factor-beta (TGFβ) induce the proliferation of myeloid-derived suppressor cells (MDSCs) and the polarization of tumor-associated macrophages (TAMs) towards the M2 subtype. MDSCs exert immunosuppressive functions, such as the inhibition of T-effector and natural killer (NK)-cells. The inflammatory cytokines cooperate to induce cyclooxygenase-2 (COX-2) and lead to prostaglandin E2 (PGE2) synthesis, which limits T-cell recruiting at tumor sites. M2 macrophages promote immunosuppression by producing cytokines (e.g., IL-1R, IL-10, C-C Motif Chemokine Ligand [CCL]17, CCL20, CCL22) that inhibit T-effectors proliferation and enhance Tregs function.
OC encompasses a heterogeneous group of malignancies that in over 95% of cases have an epithelial origin and are more frequently represented by high grade serous ovarian carcinoma (HGSOC) (70% of cases), followed by endometrioid ovarian cancer (EOC) (10%), clear cell OC (ccOC) (10%), low-grade serous OC (LGSOC, less than 5%), and mucinous OC (MOC, around 3%) [3]. Among them, the ccOC seems to be the most immunogenic: it more frequently carries the DNA microsatellite instability (MSI), has higher CD8+ tumor-infiltrating lymphocites (TILs), CD8+/CD4+ ratio, and higher PD-L1 levels [57][58]. Effectively, it is five times more responsive to ICIs than other OC subtypes [19]. Even among HGSOC, at least four different genomic classes were identified in The Cancer Genomic Atlas registry, differing for immunoreactivity. A unique subtype expresses genes related to immune sensitivity such as Toll-like receptor (TLR), TNF and is characterized by higher TILs infiltration [59][60]. Moreover, proteomics studies showed that the four subclasses of HSGOC are characterized by different expressions of proteins involved in DNA replication, ECM and cellular interaction, and cytokine signaling that contributes to immune responsiveness [61]. In our opinion, the different ICIs response observed among OC patients is rooted in the inter-tumor heterogeneity. Therefore, a deeper insight into the genomics characteristics of OC and their relationship with the immunological profile could allow us to better clarify the predictive factors for ICIs response. Ideally, specific immunogenomic scores could be developed for more accurate patients selection.
OC has been indicated as potentially more immune responsive when carrying BRCA mutations or homologous recombination deficiency (HRD). In fact, the impaired DNA repair leads to neo-antigens production, resulting in a higher tumor mutational burden (TMB) (even if <10 mutations per megabase are usually detected) and recruiting TILs at tumor sites. However, HRD or BRCA mutations were not linked to a higher sensitivity to ICIs in the IMagyn050 nor in the Javelin Ovarian 100 trials [23][27]. BRCA-mutant/HRD OC is associated with higher CD3+ and CD8+ TILs, PD1/PD-L1 levels, and genes related to cytotoxicity, such as T-Cell Receptor (TCR), γ-IFN, and TNF-Receptor pathway [62][63][64][65]. As proof of this, in the NCT02484404 trial, durvalumab plus olaparib determined a longer PFS in case of increased γ-IFN production [33]. Another mechanism of immune responsiveness is represented by the mismatch repair (MMR) deficiency, harboring the DNA MSI. MSI tumors produce neo-antigens, with a 10–100-fold higher TMB than MS stable (MSS)-tumors, resulting in high immunogenicity. Some genes triggering MSI were also identified in a percentage ranging from 17% to 59% of OC (more commonly in non-serous subtypes): the oncosuppressor TP53; Dihydropyrimidinase-related protein (DPYSL)-2, involved in microtubules function; Alpha Kinase (ALPK)-2, with a role in apoptosis and DNA repair [66]. In Lynch syndrome, a germline mutation of the MMR genes MutL homolog (MLH)-1, MutS homolog (MSH)-2 and -6, PMS1 homolog (PMS)-2 leads to an increased risk to develop some cancer subtypes, including OC [67]. Therefore, these tumors may be good candidates for ICIs treatment. Other genes could be involved in ICI response, justifying the different results observed among OC patients. The SWItch/Sucrose Non-Fermentable (SWI/SNF) complex consists of around 15 subunits, acting as a chromatin remodeler. In other tumor subtypes, the loss of function of the SWI/SNF complex predicts ICI response, increasing MMR deficiency, TMB, and neo-antigens production [68]. SWI/SNF complex mutations were frequently detected in OC [69]. We can assume that genetic diversity contributes to different ICI responses among OC patients. A more extensive genetic characterization could allow more accurate identification of responders and non-responders.
The possible relationship between platinum- and immunotherapy-sensitivity/resistance is also a field that merits further investigation [70]. A series of genetic and epigenetic elements were identified to drive platinum response: alterations of p53, specific microRNAs, elements driving the epithelial-to-mesenchymal transition (EMT), HRD, and BRCA mutations [71]. Since BRCA mutation and HRD were proposed to correlate with platinum sensitivity in contemporary deficient nucleotide excision repair, the co-administration of PARPis and ICIs in PS-ROC could result in higher ORR and survival rates [29][30][31]. PARPis enhance ICIs activity because they induce the release of neoantigens, increasing the TMB, promote PD-L1 expression, and directly activate the IFN genes; however, this was determined in OC [17][29][30][31][72][73]. Many ongoing trials are addressing this combination strategy in the advanced setting.
As ICIs monotherapies showed only minimal results in terms of response rate and survival in OC, the combination with agents with different mechanisms of action appears a promising strategy to increase efficacy. Although chemotherapy represents a cornerstone in the treatment of advanced OC, it was historically perceived to play an immunosuppressive role. On the contrary, more recently, it has emerged that platinum derivatives promote APCs and their function, activating the immune response [74][75][76][77]. Doxorubicin plays an immunomodulatory effect, reducing the immunosuppressive state and improving tumor sensitivity to NK and CD8+ T-cells [78]. Low-dose cyclophosphamide also holds immunomodulatory properties, such as Tregs reduction and CD8+ cells induction [79][80]. However, the studies conducted so far did not lead to survival improvements. Besides the immunological potential, timing and schedule should be more deeply investigated and optimized for improving efficacy. The combination of ICIs and anti-VEGF agents seems attractive because the anti-angiogenic drugs directly influence OC TME [20][31][32][81][82][83]. Other combinations with multikinase inhibitors targeting VEGF/VEGFRpathway, such as cabozantinib or lenvatinib, are now under evaluation. The association with other agents with immunotherapeutics role, such as the anti-Lymphocyte-activation gene 3 (LAG-3) Relatlimab, as well as monoclonal antibodies such as the anti-Cluster of differentiation (CD)27 Varlilumab, the anti-CD47 Magrolimab, is under investigation. Actually, overcoming the immunosuppressive pathways in the TME could represent a complementary way to potentiate ICIs effect on the immune system. Therapeutic vaccines were administered in OC, inducing cellular and humoral responses but rarely survival improvement as monotherapies [84]. Hence, several tumor-associated antigens were found in OC, such as p53, folate receptor (FR), New York Esophageal Squamous Cell Carcinoma-1 (NY-ESO-1), and Ca125 [85][86][87][88]. Therefore, combinations of ICIs and vaccines need to be explored. New approaches such as autologous TILs, cancer cell therapy, and adoptive cell therapy (ACT) also represent future possibilities for improving ICIs efficacy.
Currently, a uniformly accepted predictive role of PD-L1 for ICIs response was not yet identified in solid tumors, including OC. PD-L1 expression varies between primary tumors and metastases, implying heterogeneity [89]. However, even if PD-L1 positivity was retrieved in around 1/3 OCs, the clinical impact was not elucidated, with conflicting results regarding the association with higher tumor stage/grade or shorter survival [90][91][92][93][94]. Indeed, some of the published trials reported better results for PD-L1 positive than PD-L1 negative patients [12][13][27]. In other studies, PD-L1 positivity was not predictive of ICIs response [19][20]. Recent research has focused on the post-transcriptional modifications of PD1, and even more PD-L1, which N-glycosylation of specific sites functionally modulates. PD-L1 and PD1 N-glycosylation ensure stability, prevents clearance, and influences mutual interactions [95][96]. The N-glycosylation of the PD1/PD-L1 receptors and its aberrations should be better investigated as possible immune resistance mechanisms in OC since specific glycoproteomic signatures were found in HGSOC: the immunoreactive subtype was richer in mannose than the mesenchymal, which was mainly fucosylated [97]. Moreover, it was evidenced that the antibodies used in the immunohistochemical analysis for PD-L1 accessed the highly glycosylated PD-L1 with difficulty, resulting in a certain percentage of PD-L1 false-negative results partially explaining ICIs efficacy also in PD-L1 negative patients [98]. More profound knowledge of the post-transcriptional status of PD1/PD-L1 and the search for biomarkers with a predictive role for ICIs’ efficacy is warranted to ensure the best patient selection.
References
- Globocan 2020. Ovary. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/25-Ovary-fact-sheet.pdf (accessed on 15 June 2021).
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian Cancer Statistics, 2018. CA A Cancer J. Clin. 2018, 68, 284–296.
- Prat, J.; D’Angelo, E.; Espinosa, I. Ovarian Carcinomas: At Least Five Different Diseases with Distinct Histological Features and Molecular Genetics. Hum. Pathol. 2018, 80, 11–27.
- Piccart, M.J. Randomized Intergroup Trial of Cisplatin-Paclitaxel vs. Cisplatin-Cyclophosphamide in Women with Advanced Epithelial Ovarian Cancer: Three-Year Results. J. Natl. Cancer Inst. 2000, 92, 699–708.
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R. Phase III Trial of Carboplatin and Paclitaxel Compared with Cisplatin and Paclitaxel in Patients with Optimally Resected Stage III Ovarian Cancer: A Gynecologic Oncology Group Study. JCO 2003, 21, 3194–3200.
- Neijt, J.P.; Engelholm, S.A.; Tuxen, M.K.; Sørensen, P.G.; Hansen, M.; Sessa, C.; de Swart, C.A.M.; Hirsch, F.R.; Lund, B.; van Houwelingen, H.C. Exploratory Phase III Study of Paclitaxel and Cisplatin vs. Paclitaxel and Carboplatin in Advanced Ovarian Cancer. JCO 2000, 18, 3084–3092.
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. N. Engl. J. Med. 1996, 334, 1–6.
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2484–2496.
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505.
- Robert, C. A Decade of Immune-Checkpoint Inhibitors in Cancer Therapy. Nat. Commun. 2020, 11, 3801.
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. JCO 2015, 33, 1974–1982.
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.D.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M.; et al. Antitumor Activity and Safety of Pembrolizumab in Patients with Advanced Recurrent Ovarian Cancer: Results from the Phase II KEYNOTE-100 Study. Ann. Oncol. 2019, 30, 1080–1087.
- Matulonis, U.A.; Shapira, R.; Santin, A.; Lisyanskaya, A.S.; Pignata, S.; Vergote, S.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Sehouli, J.; et al. Final Results from the KEYNOTE-100 Trial of Pembrolizumab in Patients with Advanced Recurrent Ovarian Cancer. JCO 2020, 38, 6005.
- Varga, A.; Piha-Paul, S.; Ott, P.A.; Mehnert, J.M.; Berton-Rigaud, D.; Morosky, A.; Yang, P.; Ruman, J.; Matei, D. Pembrolizumab in Patients with Programmed Death Ligand 1–Positive Advanced Ovarian Cancer: Analysis of KEYNOTE-028. Gynecol. Oncol. 2019, 152, 243–250.
- Matulonis, U.A.; Barry, W.; Penson, R.T.; Campos, S.M.; Krasner, C.; Liu, J.F. Phase II Study of Pembrolizumab (Pembro) Combined with Pegylated Liposomal Doxorubicin (PLD) for Recurrent Platinum-Resistant Ovarian, Fallopian Tube or Peritoneal Cancer. Gynecol. Oncol. 2018, 149, 24.
- Zsiros, E.; Lynam, S.; Attwood, K.M.; Wang, C.; Chilakapati, S.; Gomez, E.C.; Liu, S.; Akers, S.; Lele, S.; Frederick, P.J.; et al. Efficacy and Safety of Pembrolizumab in Combination with Bevacizumab and Oral Metronomic Cyclophosphamide in the Treatment of Recurrent Ovarian Cancer: A Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2021, 7, 78.
- Konstantinopoulos, P.A.; Waggoner, S.; Vidal, G.A.; Mita, M.; Moroney, J.W.; Holloway, R.; Van Le, L.; Sachdev, J.C.; Chapman-Davis, E.; Colon-Otero, G.; et al. Single-Arm Phases 1 and 2 Trial of Niraparib in Combination with Pembrolizumab in Patients with Recurrent Platinum-Resistant Ovarian Carcinoma. JAMA Oncol. 2019, 5, 1141.
- Hamanishi, J.; Mandai, M.; Ikeda, T.; Minami, M.; Kawaguchi, A.; Murayama, T.; Kanai, M.; Mori, Y.; Matsumoto, S.; Chikuma, S.; et al. Safety and Antitumor Activity of Anti–PD-1 Antibody, Nivolumab, in Patients with Platinum-Resistant Ovarian Cancer. JCO 2015, 33, 4015–4022.
- Zamarin, D.; Burger, R.A.; Sill, M.W.; Powell, D.J.; Lankes, H.A.; Feldman, M.D.; Zivanovic, O.; Gunderson, C.; Ko, E.; Mathews, C.; et al. Randomized Phase II Trial of Nivolumab vs. Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. JCO 2020, 38, 1814–1823.
- Liu, J.F.; Herold, C.; Gray, K.P.; Penson, R.T.; Horowitz, N.; Konstantinopoulos, P.A.; Castro, C.M.; Hill, S.J.; Curtis, J.; Luo, W.; et al. Assessment of Combined Nivolumab and Bevacizumab in Relapsed Ovarian Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 1731.
- Omatsu, K.; Hamanishi, J.; Katsumata, N.; Nishio, S.; Sawada, K.; Takeuchi, S.; Aoki, D.; Fujiwara, K.; Sugiyama, T.; Konishi, I. 807O Nivolumab vs. Gemcitabine or Pegylated Liposomal Doxorubicin for Patients with Platinum-Resistant (Advanced or Recurrent) Ovarian Cancer: Open-Label, Randomized Trial in Japan (NINJA Trial). Ann. Oncol. 2020, 31, 611.
- Disis, M.L.; Taylor, M.H.; Kelly, K.; Beck, J.T.; Gordon, M.; Moore, K.M.; Patel, M.R.; Chaves, J.; Park, H.; Mita, A.C.; et al. Efficacy and Safety of Avelumab for Patients with Recurrent or Refractory Ovarian Cancer: Phase 1b Results from the JAVELIN Solid Tumor Trial. JAMA Oncol. 2019, 5, 393.
- Ledermann, J.A.; Colombo, N.; Oza, A.M.; Fujiwara, K.; Birrer, M.J.; Randall, L.M.; Poddubskaya, E.V.; Scambia, G.; Shparyk, Y.V.; Lim, M.C.; et al. Avelumab in Combination with and/or Following Chemotherapy vs Chemotherapy Alone in Patients with Previously Untreated Epithelial Ovarian Cancer: Results from the Phase 3 Javelin Ovarian 100 Trial. Gynecol. Oncol. 2020, 159, 13–14.
- NCT03642132: Avelumab and Talazoparib in Untreated Advanced Ovarian Cancer (JAVELIN OVARIAN PARP 100). Available online: https://clinicaltrials.gov/ct2/show/NCT03642132 (accessed on 15 June 2021).
- Pujade-Lauraine, E.; Fujiwara, K.; Ledermann, J.A.; Oza, A.M.; Kristeleit, R.S.; Ray-Coquard, I.L.; Richardson, G.E.; Sessa, C.; Yonemori, K.; Banerjee, S.; et al. Avelumab Alone or in Combination with Pegylated Liposomal Doxorubicin vs. Pegylated Liposomal Doxorubicin Alone in Platinum-Resistant or Refractory Epithelial Ovarian Cancer: Primary and Biomarker Analysis of the Phase III JAVELIN Ovarian 200 Trial. Gynecol. Oncol. 2019, 154, 21–22.
- Cadoo, K.A.; Meyers, M.L.; Burger, R.A.; Armstrong, D.K.; Penson, R.T.; Gordon, M.S.; Fleming, G.F.; Moroney, J.W.; Hamilton, E.P.; Duska, L.R.; et al. A Phase II Randomized Study of Avelumab plus Entinostat vs. Avelumab plus Placebo in Patients (Pts) with Advanced Epithelial Ovarian Cancer (EOC). JCO 2019, 37, 5511.
- Moore, K.N.; Bookman, M.; Sehouli, J.; Miller, A.; Anderson, C.; Scambia, G.; Myers, T.; Taskiran, C.; Robison, K.; Mäenpää, J.; et al. Atezolizumab, Bevacizumab, and Chemotherapy for Newly Diagnosed Stage III or IV Ovarian Cancer: Placebo-Controlled Randomized Phase III Trial (IMagyn050/GOG 3015/ENGOT-OV39). JCO 2021, 39, 1842–1855.
- O’Cearbhaill, R.E.; Wolfer, A.; Disilvestro, P.; O’Malley, D.M.; Sabbatini, P.; Shohara, L.; Schwarzenberger, P.O.; Ricciardi, T.; Macri, M.; Ryan, A.; et al. A Phase I/II Study of Chemo-Immunotherapy with Durvalumab (Durva) and Pegylated Liposomal Doxorubicin (PLD) in Platinum-Resistant Recurrent Ovarian Cancer (PROC). Ann. Oncol. 2018, 29, 337.
- Drew, Y.; de Jonge, M.; Hong, S.H.; Park, Y.H.; Wolfer, A.; Brown, J.; Ferguson, M.; Gore, M.E.; Alvarez, R.H.; Gresty, C.; et al. An Open-Label, Phase II Basket Study of Olaparib and Durvalumab (MEDIOLA): Results in Germline BRCA -Mutated ( GBRCA m) Platinum-Sensitive Relapsed (PSR) Ovarian Cancer (OC). Gynecol. Oncol. 2018, 149, 246–247.
- Drew, Y.; Kaufman, B.; Banerjee, S.; Lortholary, A.; Hong, S.H.; Park, Y.H.; Zimmermann, S.; Roxburgh, P.; Ferguson, M.; Alvarez, R.H.; et al. Phase II study of olaparib 1 durvalumab (MEDIOLA): Updated Results in Germline BRCA-Mutated Platinum-Sensitive Relapsed (PSR) Ovarian Cancer (OC). Ann. Oncol. 2019, 30, 485–486.
- Drew, Y.; Penson, R.T.; O’Malley, D.M.; Kim, J.-W.; Zimmermann, S.; Roxburgh, P.; Sohn, J.; Stemmer, S.M.; Bastian, S.; Ferguson, M.; et al. 814MO Phase II Study of Olaparib (O) plus Durvalumab (D) and Bevacizumab (B) (MEDIOLA): Initial Results in Patients (Pts) with Non-Germline BRCA-Mutated (Non-GBRCAm) Platinum Sensitive Relapsed (PSR) Ovarian Cancer (OC). Ann. Oncol. 2020, 31, 615–616.
- Lee, J.M.; Cimino-Mathews, A.; Peer, C.J.; Zimmer, A.; Lipkowitz, S.; Annunziata, C.M.; Cao, L.; Harrell, M.I.; Swisher, E.M.; Houston, N.; et al. Safety and Clinical Activity of the Programmed Death-Ligand 1 Inhibitor Durvalumab in Combination with Poly (ADP-Ribose) Polymerase Inhibitor Olaparib or Vascular Endothelial Growth Factor Receptor 1–3 Inhibitor Cediranib in Women’s Cancers: A Dose-Escalation, Phase I Study. JCO 2017, 35, 2193–2202.
- Lampert, E.J.; Zimmer, A.; Padget, M.; Cimino-Mathews, A.; Nair, J.R.; Liu, Y.; Swisher, E.M.; Hodge, J.W.; Nixon, A.B.; Nichols, E.; et al. Combination of PARP Inhibitor Olaparib, and PD-L1 Inhibitor Durvalumab, in Recurrent Ovarian Cancer: A Proof-of-Concept Phase II Study. Clin. Cancer Res. 2020, 26, 4268–4279.
- NCT01611558: Phase II Study of Ipilimumab Monotherapy in Recurrent Platinum-Sensitive Ovarian Cancer-Study Results. Available online: https://clinicaltrials.gov/ct2/show/results/NCT01611558 (accessed on 15 June 2021).
- Gaillard, S.; Berg, M.; Harrison, J.; Huang, P.; Leatherman, J.M.; Doucet, M.; Sen, R.; Suru, A.; Cai, H.; Durham, J.N.; et al. A Clinical Study of Tremelimumab Alone or in Combination with Olaparib in Patients with Advanced Epithelial Ovarian Cancer. JCO 2020, 38, 6045.
- Adams, S.F.; Rixe, O.; Lee, J.-H.; McCance, D.J.; Westgate, S.; Eberhardt, S.C.; Rutledge, T.; Muller, C. Phase I Study Combining Olaparib and Tremelimumab for the Treatment of Women with BRCA-Deficient Recurrent Ovarian Cancer. JCO 2017, 35, 17052.
- Hartnett, E.G.; Knight, J.; Radolec, M.; Buckanovich, R.J.; Edwards, R.P.; Vlad, A.M. Immunotherapy Advances for Epithelial Ovarian Cancer. Cancers 2020, 12, 3733.
- Ning, F.; Cole, C.B.; Annunziata, C.M. Driving Immune Responses in the Ovarian Tumor Microenvironment. Front. Oncol. 2021, 10, 604084.
- Yang, Y.; Yang, Y.; Yang, J.; Zhao, X.; Wei, X. Tumor Microenvironment in Ovarian Cancer: Function and Therapeutic Strategy. Front. Cell Dev. Biol. 2020, 8, 758.
- Konstantinopoulos, P.A.; Cannistra, S.A. Immune Checkpoint Inhibitors in Ovarian Cancer: Can We Bridge the Gap Between IMagynation and Reality? JCO 2021, 10, 1833–1838.
- Kapka-Skrzypczak, L.; Wolinska, E.; Szparecki, G.; Czajka, M.; Skrzypczak, M. The Immunohistochemical Analysis of Membrane-Bound CD55, CD59 and Fluid-Phase FH and FH-like Complement Inhibitors in Cancers of Ovary and Corpus Uteri Origin. Cent. Eur. J. Immunol. 2015, 3, 349–353.
- Wouters, M.; Dijkgraaf, E.; Kuijjer, M.; Jordanova, E.; Hollema, H.; Welters, M.; van der Hoeven, J.; Daemen, T.; Kroep, J.; Nijman, H.; et al. Interleukin-6 Receptor and Its Ligand Interleukin-6 Are Opposite Markers for Survival and Infiltration with Mature Myeloid Cells in Ovarian Cancer. OncoImmunology 2014, 3, e962397.
- Kolomeyevskaya, N.; Eng, K.H.; Khan, A.N.H.; Grzankowski, K.S.; Singel, K.L.; Moysich, K.; Segal, B.H. Cytokine Profiling of Ascites at Primary Surgery Identifies an Interaction of Tumor Necrosis Factor-α and Interleukin-6 in Predicting Reduced Progression-Free Survival in Epithelial Ovarian Cancer. Gynecol. Oncol. 2015, 138, 352–357.
- Wong, J.L.; Obermajer, N.; Odunsi, K.; Edwards, R.P.; Kalinski, P. Synergistic COX2 Induction by IFNγ and TNFα Self-Limits Type-1 Immunity in the Human Tumor Microenvironment. Cancer Immunol. Res. 2016, 4, 303–311.
- Motz, G.T.; Santoro, S.P.; Wang, L.-P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor Endothelium FasL Establishes a Selective Immune Barrier Promoting Tolerance in Tumors. Nat. Med. 2014, 20, 607–615.
- Pollard, J.W. Tumour-Educated Macrophages Promote Tumour Progression and Metastasis. Nat. Rev. Cancer 2004, 4, 71–78.
- Reinartz, S.; Schumann, T.; Finkernagel, F.; Wortmann, A.; Jansen, J.M.; Meissner, W.; Krause, M.; Schwörer, A.; Wagner, U.; Müller-Brüsselbach, S.; et al. Mixed-polarization Phenotype of Ascites-associated Macrophages in Human Ovarian Carcinoma: Correlation of CD163 Expression, Cytokine Levels and Early Relapse. Int. J. Cancer 2014, 134, 32–42.
- Yin, M.; Li, X.; Tan, S.; Zhou, H.J.; Ji, W.; Bellone, S.; Xu, X.; Zhang, H.; Santin, A.D.; Lou, G.; et al. Tumor-Associated Macrophages Drive Spheroid Formation during Early Transcoelomic Metastasis of Ovarian Cancer. J. Clin. Investig. 2016, 126, 4157–4173.
- Zhang, M.; He, Y.; Sun, X.; Li, Q.; Wang, W.; Zhao, A.; Di, W. A High M1/M2 Ratio of Tumor-Associated Macrophages Is Associated with Extended Survival in Ovarian Cancer Patients. J. Ovarian Res. 2014, 7, 19.
- Lan, C.; Huang, X.; Lin, S.; Huang, H.; Cai, Q.; Wan, T.; Lu, J.; Liu, J. Expression of M2-Polarized Macrophages Is Associated with Poor Prognosis for Advanced Epithelial Ovarian Cancer. Technol. Cancer Res. Treat. 2013, 12, 259–267.
- Yuan, X.; Zhang, J.; Li, D.; Mao, Y.; Mo, F.; Du, W.; Ma, X. Prognostic Significance of Tumor-Associated Macrophages in Ovarian Cancer: A Meta-Analysis. Gynecol. Oncol. 2017, 147, 181–187.
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35.
- Li, H.; Fan, X.; Houghton, J. Tumor Microenvironment: The Role of the Tumor Stroma in Cancer. J. Cell. Biochem. 2007, 101, 805–815.
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific Recruitment of Regulatory T Cells in Ovarian Carcinoma Fosters Immune Privilege and Predicts Reduced Survival. Nat. Med. 2004, 10, 942–949.
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular Mechanisms of Treg-Mediated T Cell Suppression. Front. Immun. 2012, 3.
- Obermajer, N.; Muthuswamy, R.; Lesnock, J.; Edwards, R.P.; Kalinski, P. Positive Feedback between PGE2 and COX2 Redirects the Differentiation of Human Dendritic Cells toward Stable Myeloid-Derived Suppressor Cells. Blood 2011, 118, 5498–5505.
- Howitt, B.E.; Strickland, K.C.; Sholl, L.M.; Rodig, S.; Ritterhouse, L.L.; Chowdhury, D.; D’Andrea, A.D.; Matulonis, U.A.; Konstantinopoulos, P.A. Clear Cell Ovarian Cancers with Microsatellite Instability: A Unique Subset of Ovarian Cancers with Increased Tumor-Infiltrating Lymphocytes and PD-1/PD-L1 Expression. OncoImmunology 2017, 6, e1277308.
- Oda, K.; Hamanishi, J.; Matsuo, K.; Hasegawa, K. Genomics to Immunotherapy of Ovarian Clear Cell Carcinoma: Unique Opportunities for Management. Gynecol. Oncol. 2018, 151, 381–389.
- Verhaak, R.G.W.; Tamayo, P.; Yang, J.-Y.; Hubbard, D.; Zhang, H.; Creighton, C.J.; Fereday, S.; Lawrence, M.; Carter, S.L.; Mermel, C.H.; et al. Prognostically Relevant Gene Signatures of High-Grade Serous Ovarian Carcinoma. J. Clin. Invest. 2012.
- Przybytkowski, E.; Davis, T.; Hosny, A.; Eismann, J.; Matulonis, U.A.; Wulf, G.M.; Nabavi, S. An Immune-Centric Exploration of BRCA1 and BRCA2 Germline Mutation Related Breast and Ovarian Cancers. BMC Cancer 2020, 20, 197.
- Zhang, H.; Liu, T.; Zhang, Z.; Payne, S.H.; Zhang, B.; McDermott, J.E.; Zhou, J.Y.; Petyuk, V.A.; Chen, L.; Ray, D.; et al. CPTAC Investigators. Integrated Proteogenomic Characterization of Human High-Grade Serous Ovarian Cancer. Cell 2016, 166, 755–765.
- Strickland, K.C.; Howitt, B.E.; Shukla, S.A.; Rodig, S.; Ritterhouse, L.L.; Liu, J.F.; Garber, J.E.; Chowdhury, D.; Wu, C.J.; D’Andrea, A.D.; et al. Association and Prognostic Significance of BRCA1/2-Mutation Status with Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes and Expression of PD-1/PD-L1 in High Grade Serous Ovarian Cancer. Oncotarget 2016, 7, 13587–13598.
- Morse, C.B.; Toukatly, M.N.; Kilgore, M.R.; Agnew, K.J.; Bernards, S.S.; Norquist, B.M.; Pennington, K.P.; Garcia, R.L.; Liao, J.B.; Swisher, E.M. Tumor Infiltrating Lymphocytes and Homologous Recombination Deficiency Are Independently Associated with Improved Survival in Ovarian Carcinoma. Gynecol. Oncol. 2019, 153, 217–222.
- McAlpine, J.N.; Porter, H.; Köbel, M.; Nelson, B.H.; Prentice, L.M.; Kalloger, S.E.; Senz, J.; Milne, K.; Ding, J.; Shah, S.P.; et al. BRCA1 and BRCA2 Mutations Correlate with TP53 Abnormalities and Presence of Immune Cell Infiltrates in Ovarian High-Grade Serous Carcinoma. Mod. Pathol. 2012, 25, 740–750.
- Gadducci, A.; Guerrieri, M.E. PARP Inhibitors Alone and in Combination with Other Biological Agents in Homologous Recombination Deficient Epithelial Ovarian Cancer: From the Basic Research to the Clinic. Crit. Rev. Oncol. Hematol. 2017, 114, 153–165.
- Deshpande, M.; Romanski, P.A.; Rosenwaks, Z.; Gerhardt, J. Gynecological Cancers Caused by Deficient Mismatch Repair and Microsatellite Instability. Cancers 2020, 12, 3319.
- Helder-Woolderink, J.M.; Blok, E.A.; Vasen, H.F.A.; Hollema, H.; Mourits, M.J.; De Bock, G.H. Ovarian Cancer in Lynch Syndrome; a Systematic Review. Eur. J. Cancer 2016, 55, 65–73.
- Shen, J.; Ju, Z.; Zhao, W.; Wang, L.; Peng, Y.; Ge, Z.; Nagel, Z.D.; Zou, J.; Wang, C.; Kapoor, P.; et al. ARID1A Deficiency Promotes Mutability and Potentiates Therapeutic Antitumor Immunity Unleashed by Immune Checkpoint Blockade. Nat. Med. 2018, 24, 556–562.
- Wang, Y.; Hoang, L.; Ji, J.X.; Huntsman, D.G. SWI/SNF Complex Mutations in Gynecologic Cancers: Molecular Mechanisms and Models. Annu. Rev. Pathol. 2020, 15, 467–492.
- Van Zyl, B.; Tang, D.; Bowden, N.A. Biomarkers of Platinum Resistance in Ovarian Cancer: What Can We Use to Improve Treatment. Endocr. Relat. Cancer 2018, 25, 303–318.
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520.
- Shen, J.; Zhao, W.; Ju, Z.; Wang, L.; Peng, Y.; Labrie, M.; Yap, T.A.; Mills, G.B.; Peng, G. PARPi Triggers the STING-Dependent Immune Response and Enhances the Therapeutic Efficacy of Immune Checkpoint Blockade Independent of BRCAness. Cancer Res. 2019, 79, 311–319.
- Vikas, P.; Borcherding, N.; Chennamadhavuni, A.; Garje, R. Therapeutic Potential of Combining PARP Inhibitor and Immunotherapy in Solid Tumors. Front. Oncol. 2020, 10, 570.
- Rébé, C.; Demontoux, L.; Pilot, T.; Ghiringhelli, F. Platinum Derivatives Effects on Anticancer Immune Response. Biomolecules 2019, 10, 13.
- Grabosch, S.; Bulatovic, M.; Zeng, F.; Ma, T.; Zhang, L.; Ross, M.; Brozick, J.; Fang, Y.; Tseng, G.; Kim, E.; et al. Cisplatin-Induced Immune Modulation in Ovarian Cancer Mouse Models with Distinct Inflammation Profiles. Oncogene 2019, 38, 2380–2393.
- Wu, X.; Feng, Q.-M.; Wang, Y.; Shi, J.; Ge, H.-L.; Di, W. The Immunologic Aspects in Advanced Ovarian Cancer Patients Treated with Paclitaxel and Carboplatin Chemotherapy. Cancer Immunol. Immunother. 2010, 59, 279–291.
- De Biasi, A.R.; Villena-Vargas, J.; Adusumilli, P.S. Cisplatin-Induced Antitumor Immunomodulation: A Review of Preclinical and Clinical Evidence. Clin. Cancer Res. 2014, 20, 5384–5391.
- Zhang, Z.; Yu, X.; Wang, Z.; Wu, P.; Huang, J. Anthracyclines Potentiate Anti-Tumor Immunity: A New Opportunity for Chemoimmunotherapy. Cancer Letters 2015, 369, 331–335.
- Madondo, M.T.; Quinn, M.; Plebanski, M. Low Dose Cyclophosphamide: Mechanisms of T-Cell Modulation. Cancer Treat. Rev. 2016, 42, 3–9.
- Mkrtichyan, M.; Najjar, Y.G.; Raulfs, E.C.; Abdalla, M.Y.; Samara, R.; Rotem-Yehudar, R.; Cook, L.; Khleif, S.N. Anti-PD-1 Synergizes with Cyclophosphamide to Induce Potent Anti-Tumor Vaccine Effects through Novel Mechanisms. Eur. J. Immunol. 2011, 41, 2977–2986.
- Ziogas, A.C.; Gavalas, N.G.; Tsiatas, M.; Tsitsilonis, O.; Politi, E.; Terpos, E.; Rodolakis, A.; Vlahos, G.; Thomakos, N.; Haidopoulos, D.; et al. VEGF Directly Suppresses Activation of T-Cells from Ovarian Cancer Patients and Healthy Individuals via VEGF Receptor Type 2. Int. J. Cancer 2012, 130, 857–864.
- Shrimali, R.K.; Yu, Z.; Theoret, M.R.; Chinnasamy, D.; Restifo, N.P.; Rosenberg, S.A. Antiangiogenic Agents Can Increase Lymphocyte Infiltration into Tumor and Enhance the Effectiveness of Adoptive Immunotherapy of Cancer. Cancer Res. 2010, 70, 6171–6180.
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340.
- Chow, S.; Berek, J.S.; Dorigo, O. Development of Therapeutic Vaccines for Ovarian Cancer. Vaccines 2020, 8, 657.
- Shih, I.; Kurman, R.J. Ovarian Tumorigenesis: A Proposed Model Based on Morphological and Molecular Genetic Analysis. Am. J. Pathol. 2004, 164, 1511–1518.
- Peoples, G.E.; Anderson, B.W.; Fisk, B.; Kudelka, A.P.; Wharton, J.T.; Ioannides, C.G. Ovarian Cancer-Associated Lymphocyte Recognition of Folate Binding Protein Peptides. Ann. Surg. Oncol. 1998, 5, 743–750.
- Odunsi, K.; Jungbluth, A.A.; Stockert, E.; Qian, F.; Gnjatic, S.; Tammela, J.; Intengan, M.; Beck, A.; Keitz, B.; Santiago, D.; et al. NY-ESO-1 and LAGE-1 Cancer-Testis Antigens Are Potential Targets for Immunotherapy in Epithelial Ovarian Cancer. Cancer Res. 2003, 63, 6076–6083.
- Felder, M.; Kapur, A.; Gonzalez-Bosquet, J.; Horibata, S.; Heintz, J.; Albrecht, R.; Fass, L.; Kaur, J.; Hu, K.; Shojaei, H.; et al. MUC16 (CA125): Tumor Biomarker to Cancer Therapy, a Work in Progress. Mol. Cancer. 2014, 13, 129.
- Parvathareddy, S.K.; Siraj, A.K.; Al-Badawi, I.A.; Tulbah, A.; Al-Dayel, F.; Al-Kuraya, K.S. Differential Expression of PD-L1 between Primary and Metastatic Epithelial Ovarian Cancer and Its Clinico-Pathological Correlation. Sci. Rep. 2021, 11, 3750.
- Wang, Q.; Lou, W.; Di, W.; Wu, X. Prognostic Value of Tumor PD-L1 Expression Combined with CD8 + Tumor Infiltrating Lymphocytes in High Grade Serous Ovarian Cancer. Int. Immunopharmacol. 2017, 52, 7–14.
- Zhu, J.; Wen, H.; Bi, R.; Wu, Y.; Wu, X. Prognostic Value of Programmed Death-Ligand 1 (PD-L1) Expression in Ovarian Clear Cell Carcinoma. J. Gynecol. Oncol. 2017, 28, e77.
- Webb, J.R.; Milne, K.; Kroeger, D.R.; Nelson, B.H. PD-L1 Expression Is Associated with Tumor-Infiltrating T Cells and Favorable Prognosis in High-Grade Serous Ovarian Cancer. Gynecol. Oncol. 2016, 141, 293–302.
- Mesnage, S.J.L.; Auguste, A.; Genestie, C.; Dunant, A.; Pain, E.; Drusch, F.; Gouy, S.; Morice, P.; Bentivegna, E.; Lhomme, C.; et al. Neoadjuvant Chemotherapy (NACT) Increases Immune Infiltration and Programmed Death-Ligand 1 (PD-L1) Expression in Epithelial Ovarian Cancer (EOC). Ann. Oncol. 2017, 28, 651–657.
- Kim, H.-S.; Kim, J.-Y.; Lee, Y.J.; Kim, S.H.; Lee, J.-Y.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T. Expression of Programmed Cell Death Ligand 1 and Immune Checkpoint Markers in Residual Tumors after Neoadjuvant Chemotherapy for Advanced High-Grade Serous Ovarian Cancer. Gynecol. Oncol. 2018, 151, 414–421.
- Li, C.W.; Lim, S.O.; Xia, W.; Lee, H.H.; Chan, L.C.; Kuo, C.W.; Khoo, K.H.; Chang, S.S.; Cha, J.H.; Kim, T.; et al. Glycosylation and Stabilization of Programmed Death Ligand-1 Suppresses T-Cell Activity. Nat. Commun. 2016, 30, 12632.
- Wang, Y.N.; Lee, H.H.; Hsu, J.L.; Yu, D.; Hung, M. The Impact of PD-L1 N-Linked Glycosylation on Cancer Therapy and Clinical Diagnosis. J. Biomed. Sci. 2020, 27, 77.
- Pan, J.; Hu, Y.; Sun, S.; Chen, L.; Schnaubelt, M.; Clark, D.; Ao, M.; Zhang, Z.; Chan, D.; Qian, J.; et al. Glycoproteomics-Based Signatures for Tumor Subtyping and Clinical Outcome Prediction of High-Grade Serous Ovarian Cancer. Nat. Commun. 2020, 11, 6139.
- Lee, H.H.; Wang, Y.N.; Xia, W.; Chen, C.H.; Rau, K.M.; Ye, L.; Wei, Y.; Chou, C.K.; Wang, S.C.; Yan, M.; et al. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell 2019, 36, 168–178.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
801
Revisions:
2 times
(View History)
Update Date:
13 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




