
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David López Iglesias | + 3518 word(s) | 3518 | 2021-07-27 11:36:15 | | | |
| 2 | Peter Tang | + 19 word(s) | 3537 | 2021-11-05 02:56:24 | | |
Video Upload Options
Intrinsic conducting polymers (CPs) have excellent electrochemical characteristics, such as tailored electrical conductivity by electronic doping, high environmental stability, and biocompatibility. This entry intend to overview the use of conducting polymers (CPs), extensively studied due to their high versatility and electrical properties, as chemical sensor arrays in electronic tongues and noses. Their performance in terms of sensitivity and other parameters will be studied based on the characteristic features of common conducting polymers, such as electrical conductivity and nanostructured morphology. Furthermore, the application of electronic devices in commercial prototypes will also be included here.
1. Introduction
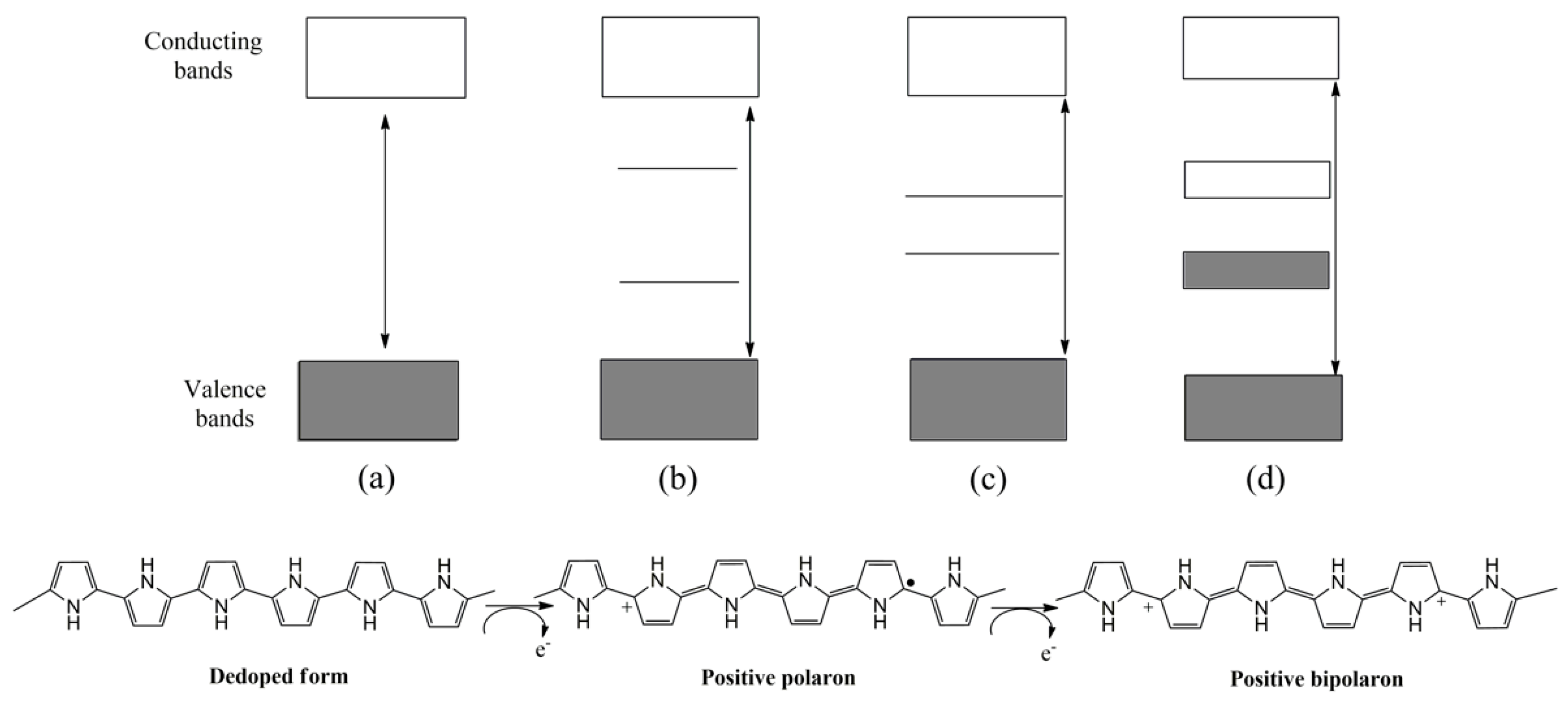
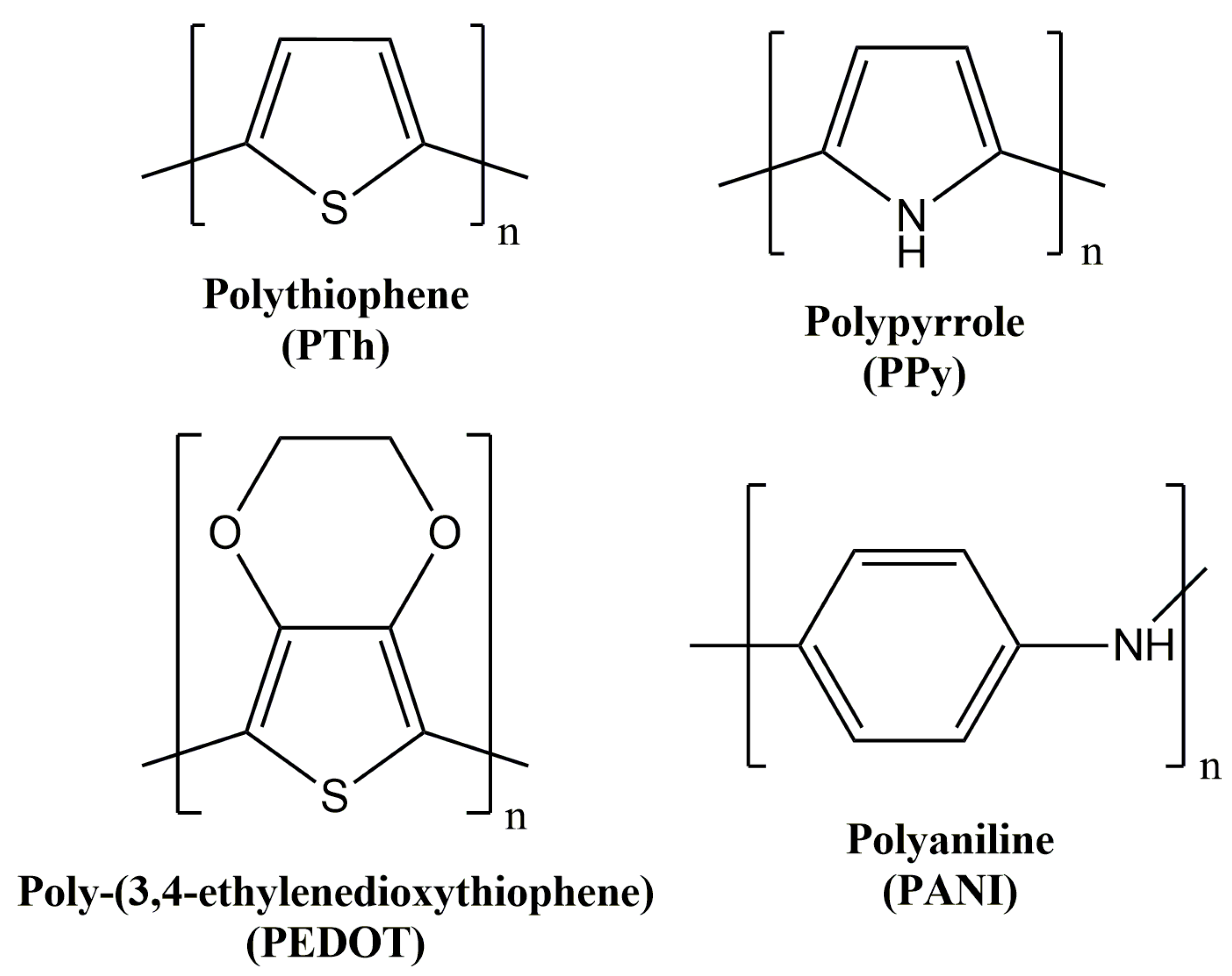
1.1. Polythiophene and Derivatives
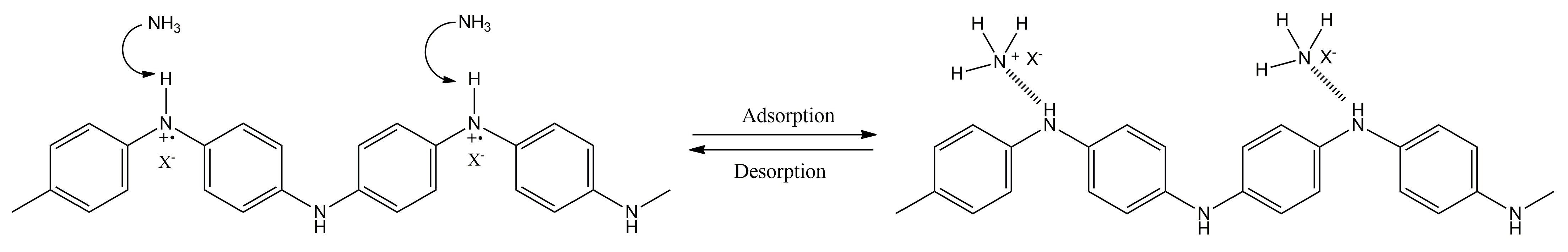
1.2. Polyaniline
1.3. Polypyrrole
1.4. Electronic Systems: Electronic Tongues and Noses
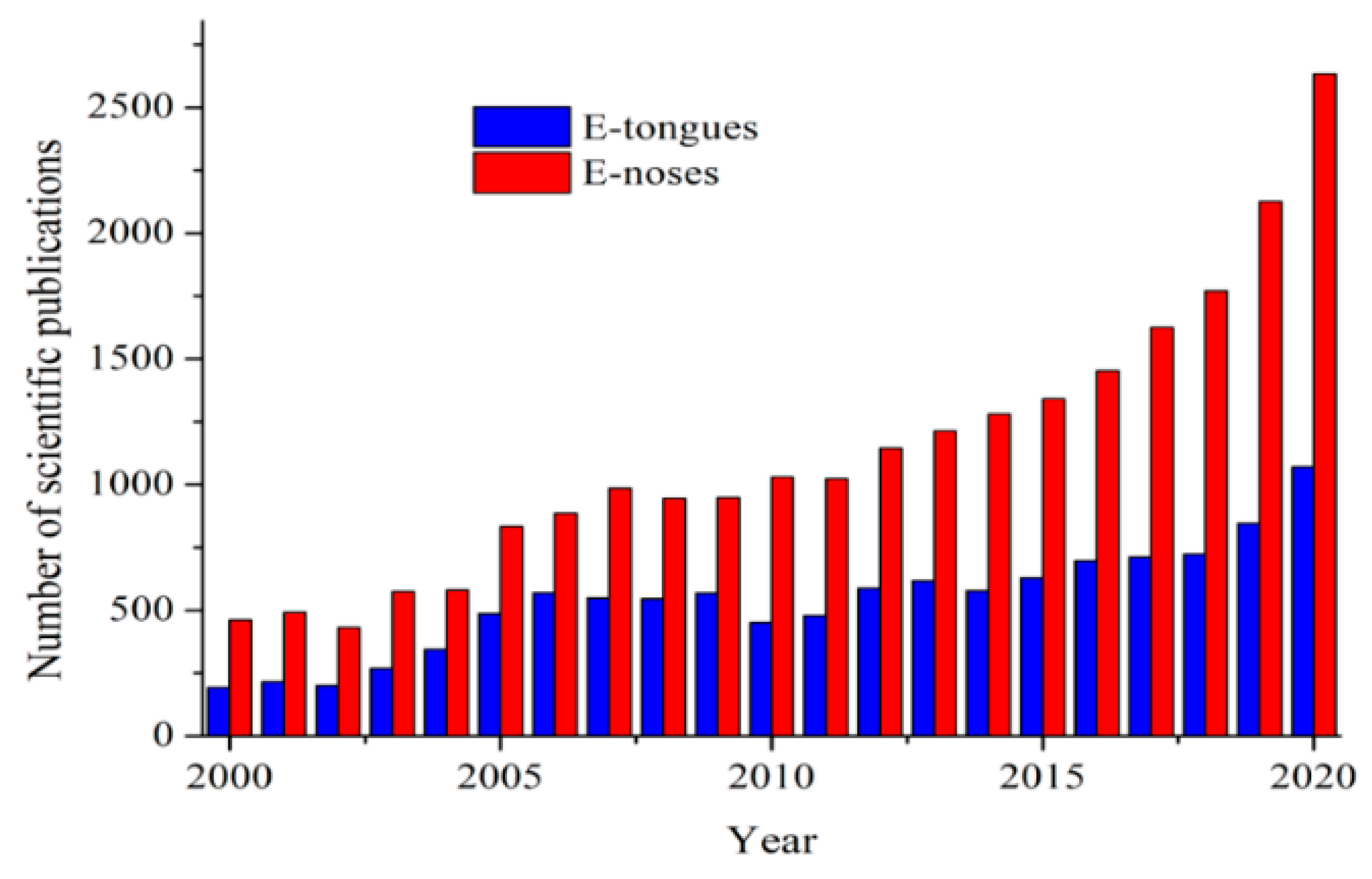
2. Electronic Tongues (E-Tongues) Based on CPs
2.1. Sensing Unit: Electrochemical Sensors
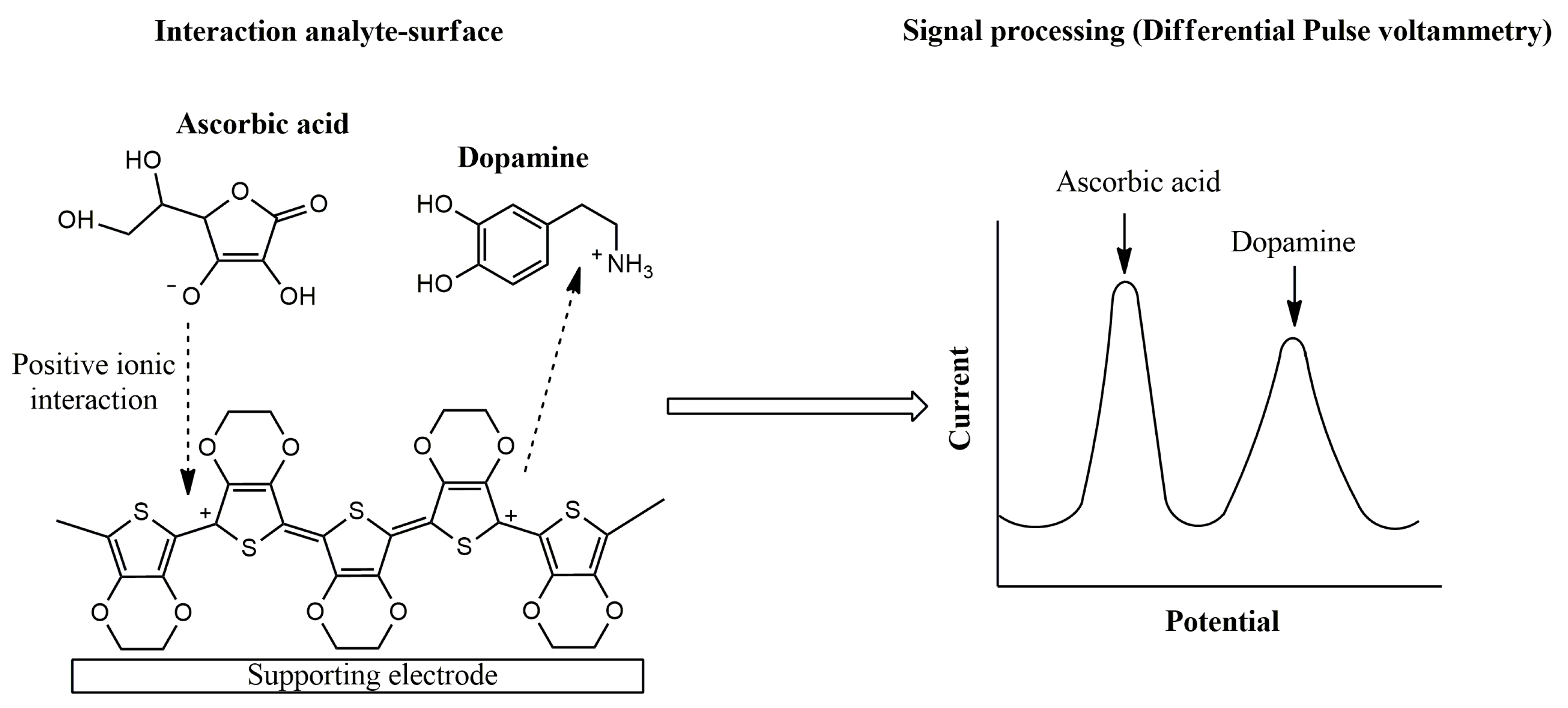
|
Electrochemical Device |
Analyte |
Working Media |
Sample |
Analytical Parameters |
Ref. |
|
|---|---|---|---|---|---|---|
|
LD (µM) |
LR (µM) |
|||||
|
PTh |
||||||
|
MWCNT/PTh/Pt |
BPA |
PBS pH 7.5 |
Water |
0.009 |
0.05–0.4 |
[56] |
|
MnO2/PTh/rGO/GCE |
MP |
PBS pH 7 |
Human urine and blood |
0.0057 |
0.5–10 |
[57] |
|
GO-4-ATP-Au-PTh/Au GCE |
Nicotine |
PBS pH 7 |
Serum, urine, cigarette |
0.17 |
1.0–30 |
[58] |
|
PTh-AgBr |
Glucose |
NaOH |
Human blood plasma |
0.31 |
4–5000 |
[59] |
|
PTh-Ag/GCE |
L-Tryp |
PBS pH 7 |
Soybeans extract |
0.020 |
0.2–400 |
[60] |
|
PEDOT |
||||||
|
PEDOT/IL/GCE |
DA |
PBS pH 7.4 |
Human urine |
0.033 |
0.2–328 |
[61] |
|
UiO-66-NH2@PEDOT/GA/GCE |
PCMC |
ABS pH 6 |
Tap water |
0.2 |
0.6–18 |
[62] |
|
PEDOT/AG/GCE |
AC |
PBS pH 7 |
Local tablets |
0.041 |
0.15–5881 |
[63] |
|
Cu2O/PEDOT/MWCNT |
Glucose |
NaOH |
Human blood serum |
0.04 |
0.495–374 |
[64] |
|
GC/PEDOT-AuNPs-SV |
CA |
PBS pH 7 |
Juice |
4.24 |
10–1000 |
[65] |
|
PEDOT-Tyr/SNG-C |
CA |
PBS pH 7 |
Wine, beer |
4.33 |
10–300 |
[66] |
|
PEDOT/PEDOT-SH/Au |
Nitrite |
PBS pH 6.9 |
Tap water, milk |
0.051 |
0.15–1000 |
[67] |
|
PEDOT/Au |
UA |
PBS pH 6.6 |
Milk |
7.0 |
6–200 |
[68] |
|
GCE/PEDOT-MC/AgNPs |
Rutin |
PBS pH 3 |
Tablets |
0.0035 |
0.005–0.5 |
[69] |
|
Pt/PEDOT-PBNPS |
H2O2 |
ABS pH 5.5 |
Human blood |
1.4 |
5–1000 |
[70] |
|
PANI |
||||||
|
Co3O4@PANINFs/GCE |
Glucose |
PBS pH 7.4 |
Human serum |
60 |
100–8000 |
[71] |
|
TiO2@PANI@Au/GCE |
Hydrazine |
NH3/NH4+ pH 9 |
Power plant sewage |
0.15 |
0.9–1200 |
[72] |
|
PANI/SnO2/GCE |
Nitrite |
PBS pH 6 |
- |
0.04 |
0.12–7777 |
[73] |
|
GCE/PANI-Fe3O4 |
DA |
PBS pH 7 |
Water |
0.176 |
0.2–2.4 |
[74] |
|
GCE/PANI-NiO |
DA |
PBS pH 7 |
Water |
0.166 |
0.2–2.4 |
[74] |
|
α-Fe2O3/PANI/GCE |
UA |
PBS pH 7 |
Human urine |
0.038 |
0.01–5 |
[75] |
|
NiO-NPs@PANINS/SPE |
Glucose |
NaOH |
Human blood serum |
0.06 |
1–3000 |
[76] |
|
MeGO/PANI |
AA |
PBS pH 7.4 |
- |
2.0 |
8–5000 |
[77] |
|
PPy |
||||||
|
Fe3O4@PPy/MWCNTs/GE |
AT |
BR pH 4 |
Serum, tablets |
0.0230 |
0.0314–201 |
[78] |
|
AuNP/PPy/GCE |
L-dopa |
PBS pH 7 |
Urine |
0.075 |
0.1–6.0 |
[79] |
|
PDA/PPy/GCE |
UA |
PBS pH 8 |
Human serum, urine |
0.11 |
0.5–40 |
[80] |
|
PGE/CuO-NPs/PPy |
TR |
PBS pH 8.5 |
Tablets |
0.001 |
0.005–380 |
[81] |
|
PPy:LAC |
Lactate |
KNO3 |
Human tear, rat blood |
81.0 |
100–10,000 |
[82] |
|
AuCu/PPy/Cu-TCCP |
H2O2 |
PBS pH 8 |
Medical H2O2 solution |
0.0067 |
0.71–24,100 |
[83] |
AA: ascorbic acid; ABS: acetic buffer solution; AC: acetaminophen; AT: atorvastatin; ATP: adenosine triphosphate; BPA: bisphenol A; BR: Britton-Robinson; CA: caffeic acid; CuO-NPs: copper oxide nanoparticles; DA: dopamine; PTh: polythiophene; GA: graphene aerogel; GCE: glassy carbon electrode; IL: ionic liquid; LAC: lactate; LD: limit of detection; LR: linear range; L-Tryp: L-tryptophan; MC: mesoporous carbon; MP: methyl parathion; MWCNT: multi-walled carbon nanotubes; PANI: polyaniline; PANINS: polyaniline nanofibers; PBNPS: Prussian blue nanoparticles; PBS: phosphate buffer solution; PCMC: p-chloromethylcresol; PEDOT: poly-(3,4-ethylenedioxythiophene); PGE: pencil graphite electrode; PPy: polypyrrole; rGO: reduced-graphene oxide; SPE: screen-printed electrode; SV: sinusoidal voltage; TCCP: meso-tetra-(4-carboxyphenyl)-substituted porphyrins; TR: tramadol; and UA: uric acid.
2.2. Analytical Application of E-Tongues
|
Sensor Array |
Sample |
Use |
Multivariate Calibration |
Ref. |
|
|---|---|---|---|---|---|
|
No CP Sensor |
CP Sensor |
||||
|
SNG-C |
PEDOT/Pt |
Musts |
Discrimination of samples collected at different ripening times |
PCA iPLS PLS |
[93] |
|
- |
PEDOT/Pt |
Red wines |
Classification of different samples and origin |
PCA PLS |
[94] |
|
Pt Au |
PEDOT/Pt |
Fruit juice |
Discrimination between samples from different fruits |
PCA PLS-LDA |
[95] |
|
IDE PA6/IDE |
PA6/PANI/IDE (0.25–5.0% PANI) |
Bovine milk |
Discrimination of samples according to tetracycline residue content |
PCA |
[96] |
|
CE AuCE rGO-CE rGO-AuCE |
PANI-CE PANI-AuCE |
Vinegar, sugar |
Multiflavor detection |
PCA |
[97] |
|
C/SPE NiO/C/SPE MWCNT/C/SPE SWCNT/C/SPE Pt |
PANI/C/SPE |
Red wine |
Phenolic content |
PCA |
[98] |
|
SWCNT/SPCE MWCNT/SPCE |
PPy-DSA/SPCE |
White wine |
Discrimination according to varietal origin |
PCA LDA |
[99] |
|
CPE-CoPc CPE-LuPc2 CPE-LuPc2 |
PPy-dopant/Au Dopant: SO4, DSA, FCN, AQDS, PWA, TSA |
Red wine |
Evaluation of chemical adulteration |
PCA PLS |
[100] |
|
GdPc2/CSPE DyPc2/CSPE CSPE |
PPy-dopant/CSPE Dopant: FeCN, NP, Mo |
Beef |
Determination of ammonia and putresceine |
PCA PLS-LDA |
[101] |
|
- |
PPy- dopant/Pt Dopant: DSA, H2SO4, FCN, AQDS, PWA, TSA |
Beer |
Evaluation of bitterness and alcoholic strength |
PCA PLS |
[102] |
|
- |
PPy-dopant/Pt Dopant: FCN, NP, PWA, H2SO4, MO, AQS |
Olive oil |
Evaluation of bitterness |
PCA PLS |
[103] |
|
- |
PPy-dopant/SPCE Dopant: DSA, SO4, FCN |
Wine |
Classification of wines according to vintage year |
PCA LDA |
[104] |
|
Graphite-epoxy PtNPs CuNPs |
PANI PPy |
Wine |
Classification of wines and recognition of the oxygenation effect |
PCA |
[105] |
AQDS: anthraquinone-2,6-disulfonic acid, disodium salt; AQS: anthraquinone-2,6-disulfonic acid; CNT: carbon nanotubes; CoPc: cobalt phthalocyanine; CPE: carbon paste electrode; CuNPs: copper nanoparticles; DSA: sodium 1-decanesulfonate; FCN: potassium hexacyanoferrate (II); IDE: interdigitated electrodes; LDA: linear discriminant analysis; LuPc2: lutetium bis-phthalocyanine; MO: sodium molybdate; MWCNT: multi-walled carbon nanotubes; PA6: polyacrilamide; PANI: polyaniline; PCA: principal component analysis; PEDOT: poly-(3,4-ethylenedioxythiophene); PLS: partial least squares regression; PPy: polypyrrole; PWA: phosphotungstic acid; PtNPS: platinum nanoparticles; rGO: reduced-graphene oxide; SNG-C: sonogel-carbon; SPCE: screen-printed-carbon electrode; SPE: screen-printed electrode; SWCNT: single-walled carbon nanotubes; and TSA: p-toluenesulfonic acid.
3. Electronic Noses (E-Noses) Based on CPs
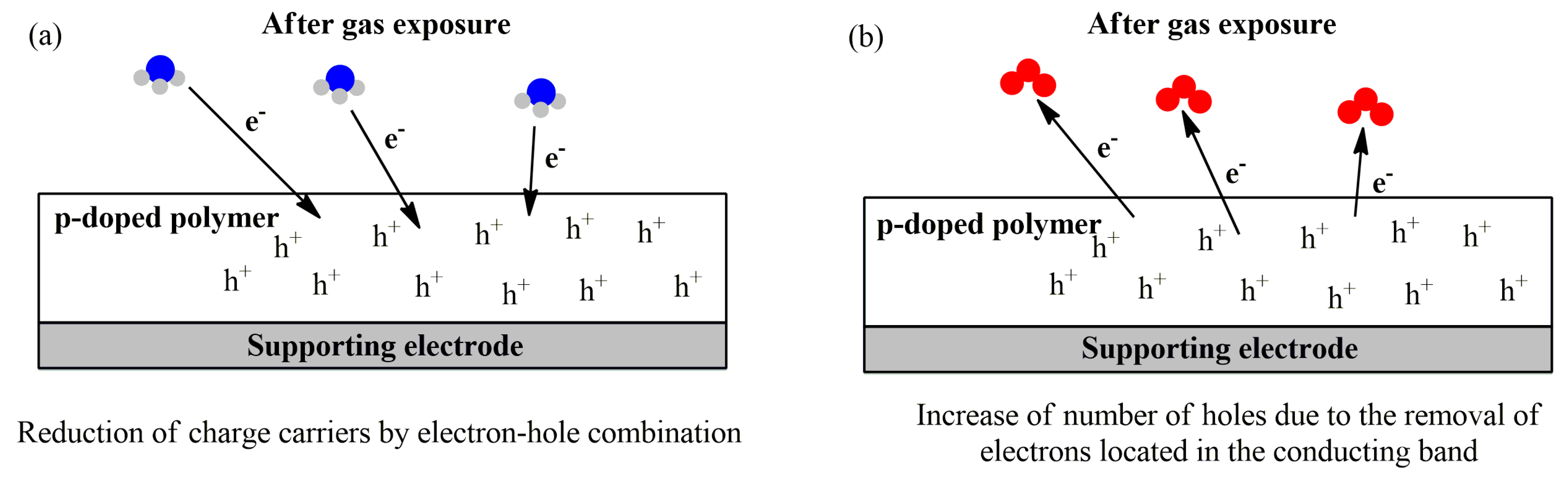
3.1. Sensing Unit: Chemiresistors

|
Gas Sensor Device |
Target Gas |
Range (ppm) |
Sensing Performance |
Ref. |
||
|---|---|---|---|---|---|---|
|
Gas Conc. (ppm) |
Recovery Time (s) |
Response Time (s) |
||||
|
SnO2/PTh |
NO2 |
10–200 |
10 |
- |
2.07 |
[116] |
|
P3CT/CNT |
NMPEA |
0.004–0.032 |
0.004 |
40 |
20 |
[117] |
|
PEDOT:PSS/FeCl3 |
NH3 |
0.2–200 |
0.5 |
- |
20 |
[118] |
|
WO3-PEDOT:PSS |
LPG |
500–3000 |
500 |
54 |
29.4 |
[119] |
|
PANI/PVDF |
NH3 |
0.2–5 |
0.2 |
235 |
174 |
[120] |
|
PANI/SnO2 |
NO2 |
5–55 |
37 |
25 |
17 |
[121] |
|
SnO2/rGO/PANI |
H2S |
0.05–10 |
2 |
78 |
82 |
[122] |
|
PANI-NF |
LPG |
100–1000 |
700 |
200 |
50 |
[123] |
|
PPy/rGO |
NH3 |
1.0–4.0 |
1.0 |
300 |
60 |
[124] |
|
PPy thin film |
NO2 |
10–100 |
10 |
374 |
218 |
[125] |
|
PPy nanoribbons |
CH3CH2OH |
- |
100 |
31 |
2 |
[126] |
|
PPy-Ag |
CH3COCH3 |
25–600 |
580 |
150 |
175 |
[127] |
|
PPy-CNT |
H2 |
1–100 |
10 |
- |
>1.0 |
[128] |
CNT: carbon nanotubes; LPG: liquified petroleum gas; NF: nickel ferrite; NMPEA: n-methylphenethylamine; P3CT: poly[3 -(6-carboxyhexyl)thiophene-2,5-diyl]; PANI: polyaniline; PEDOT: poly-(3,4-ethylenedioxythiophene); PPy: polypyrrole; PSS: poly(styrenesulfonate); PTh: polythiophene; PVDF: polyvinylidene; and r-GO: reduced-graphene oxide.
3.2. Analytical Application of E-Noses
|
PANI Sensor Array |
Sample |
Use |
Multivariate Calibration |
Ref. |
|---|---|---|---|---|
|
PANI-dopant/IDGEs Dopant: CSA, DBSA, HCl |
Strawberry Grape Apple |
Discrimination of samples according to aromatic substances |
PCA |
[139] |
|
PANI-HCl/PGIEs PANI-HCl/IDEs |
Strawberry Grape Apple |
Detection of different aromas |
PCA |
[140] |
|
PANI-dopant/IDGEs Dopant: HCl, TSA, CSA, MSA |
Cow’s estrus |
Determination of estrus times of cows |
PCA |
[141] |
|
PANI-dopant/IDEs Dopant: HCl, TSA, CSA, MSA |
Bananas |
Monitoring of bananas ripeness |
PCA |
[142] |
|
PANI-dopant/PGIEs Dopant: CSA, HCl, DBSA |
Gummy candies |
Monitoring of aromas during candy storage |
PCA |
[143] |
|
PANI-CSA/Chitosan PANI-DBSA/TiO2 PANI-DBSA/CNT |
Simulated human breath |
Preliminary diagnoses of kidney disease |
PCA LDA |
[144] |
|
PANI/AuNPs |
Human breath |
Early diagnoses of renal diseases |
PCA LDA |
[145] |
|
PANI-dopant/MWCNT PANI-dopant/GO Dopant: CSA, DBSA, HCl |
Essential oils |
Determination of quality of essential oils |
PCA |
[146] |
CSA: camphorsulfonic acid; DBSA: dodecylbenzenesulfonic acid; GO: graphene oxide; IDE: interdigitated electrode; MSA: methanesulfonic acid; MWCNT: multi-walled carbon nanotubes; PANI: polyaniline; and TSA: p-toluene sulfonic acid.
4. Future perspectives: integration of E-tongues and E-noses in commercial systems
It is not ambitious to think that the analytical applications of E-Tongues/Noses possess a great impact, not only in the foodstuff ambit but also in the health and environmental sector. Besides, this impact is rising sharply, reflecting the great need in society for these devices. Therefore, their implementation in commercial devices is exceedingly pursued by many sensor companies. Currently, there are some examples of its commercialization.
4.1 Commercial prototypes of E-tongues
Concerning E-tongues, Alpha M.O.S and Insent Inc. offers two models (αAstree and TS-5000Z, respectively) that have been used in the evaluation of food quality in the last decade[147][148][149][150][151]. Other laboratory prototypes were also employed for pharmaceutical analysis, providing very satisfactory results, as those obtained with commercial systems[152].
4.2 Commercial prototypes of E-noses
Regarding E-noses, a commercial system containing several conducting polymers as sensor arrays (Cyranose 320®), offered by Sensigent, was employed in the screening of several diseases (breast and lung cancer[153][154][155], asthma[156][157] and amyotrophic lateral sclerosis[158], among others), identification of foodstuffs (rice, wines[159] and fruits[160]) and classification of road asphalt samples[161][162]. Additionally, fecal VOCs can be inspected as well, informing about the microbial enterotype of infants[163]. Other companies also supply E-noses. For example, AromaScan A32S® (Osmetech Inc.) provides useful information about the diagnose of urban trees, being able to discriminate VOCs from healthy and decay woody samples[164] and the assessment of the quality of catfish meat[165]. In this work, off-flavour in catfish filets can be identified from good-flavour ones by means of PCA. Notably, the new device tested displayed promising features for the analysis of commercial beverages[166].
4.3 Final remarks: challenges of electrochemical/gas sensing devices
Despite the excellent analytical results provided at laboratory scale in food, pharmaceutical and medical sectors, only some timid examples can be found commercially available. In our modest opinion, the inclusion of CPs and their development may pave the way to keep growing and reach the desired applicability of E-tongues and E-noses systems. Nowadays, in order to climb up into higher technological readiness levels (TRLs), the developed devices must be able to perform reliable, robust, fast, accurate and in-situ measurements using diverse samples, by using a non-complex, low cost and portable instrumentation. The stability of the conducting coatings is another issue to take into account, since the repeatability of the responses provided with the devices can be affected. The conducting film may be passivated after performing successive electrochemical assays, as well as film overoxidation can take place at high potentials. Furthermore, stability can be affected by swelling/deswelling phenomena. With the aim to minimize these factors, several parameters, including analyte concentration, film characteristics (e.g thickness and morphology) and instrumental conditions should be carefully controlled. Further research in this sense is under study to accomplish all the commercial requirements mentioned.
References
- Bruna Hryniewicz; Elisa Orth; Marcio Vidotti; Enzymeless PEDOT-based electrochemical sensor for the detection of nitrophenols and organophosphates. Sensors and Actuators B: Chemical 2018, 257, 570-578, 10.1016/j.snb.2017.10.162.
- Nahid Shoaie; Maryam Daneshpour; Mostafa Azimzadeh; Sara Mahshid; Seyyed Mehdi Khoshfetrat; Fatemeh Jahanpeyma; Alieh Gholaminejad; Kobra Omidfar; Mehdi Foruzandeh; Electrochemical sensors and biosensors based on the use of polyaniline and its nanocomposites: a review on recent advances. Microchimica Acta 2019, 186, 465, 10.1007/s00604-019-3588-1.
- Aminur Rahman; Pankaj Kumar; Deog-Su Park; Yoon-Bo Shim; Electrochemical Sensors Based on Organic Conjugated Polymers. Sensors 2008, 8, 118-141, 10.3390/s8010118.
- Yung Cheng Wong; Bee Chin Ang; A. S. M. A. Haseeb; Aainaa Aqilah Baharuddin; Yew Hoong Wong; Review—Conducting Polymers as Chemiresistive Gas Sensing Materials: A Review. Journal of The Electrochemical Society 2019, 167, 037503, 10.1149/2.0032003jes.
- Yu Lin; Yongmin Zhao; Qian Xin; Chunping Jiang; Aimin Song; Electrical control of the optical dielectric properties of PEDOT:PSS thin films. Optical Materials 2020, 108, 110435, 10.1016/j.optmat.2020.110435.
- Irma Zulayka Mohamad Ahad; Sulaiman Wadi Harun; Seng Neon Gan; Sook Wai Phang; Polyaniline (PAni) optical sensor in chloroform detection. Sensors and Actuators B: Chemical 2018, 261, 97-105, 10.1016/j.snb.2018.01.082.
- Sara A. AlQarni; Mahmoud A. Hussein; Aisha A. Ganash; Anish Khan; Composite Material–Based Conducting Polymers for Electrochemical Sensor Applications: a Mini Review. BioNanoScience 2020, 10, 351-364, 10.1007/s12668-019-00708-x.
- Francesco Greco; Alessandra Zucca; Silvia Taccola; Arianna Menciassi; Toshinori Fujie; Hiroki Haniuda; Shinji Takeoka; Paolo Dario; Virgilio Mattoli; Ultra-thin conductive free-standing PEDOT/PSS nanofilms. Soft Matter 2011, 7, 10642-10650, 10.1039/c1sm06174g.
- Rui Chen; Shanshan Chen; Yongli Zhou; Zhouyin Wei; Haiyan Wang; Yujie Zheng; Meng Li; Kuan Sun; Yongfang Li; Unsubstituted Polythiophene Film Deposited via In-Situ Sequential Solution Polymerization for Chemo-/Electrochromism. Macromolecules 2020, 53, 4247-4254, 10.1021/acs.macromol.0c00297.
- Rui Chen; Kuan Sun; Qi Zhang; Yongli Zhou; Meng Li; Yuyang Sun; Zhou Wu; Yuyang Wu; Xinlu Li; Jialei Xi; et al.Chi MaYiyang ZhangJianyong Ouyang Sequential Solution Polymerization of Poly(3,4-ethylenedioxythiophene) Using V2O5 as Oxidant for Flexible Touch Sensors. iScience 2019, 12, 66-75, 10.1016/j.isci.2019.01.003.
- Vessela Tsakova; Renato Seeber; Conducting polymers in electrochemical sensing: factors influencing the electroanalytical signal. Fresenius' Journal of Analytical Chemistry 2016, 408, 7231-7241, 10.1007/s00216-016-9774-7.
- Kangkang Zhou; Kun Dai; Chuntai Liu; Changyu Shen; Flexible conductive polymer composites for smart wearable strain sensors. SmartMat 2020, 1, e1010, 10.1002/smm2.1010.
- Yueyue Qian; Chuang Ma; Shupeng Zhang; Juanjuan Gao; Maoxiang Liu; Kangjun Xie; Shuang Wang; Kuan Sun; Haiou Song; High performance electrochemical electrode based on polymeric composite film for sensing of dopamine and catechol. Sensors and Actuators B: Chemical 2018, 255, 1655-1662, 10.1016/j.snb.2017.08.174.
- Abhishek Kumar Mishra; Conducting Polymers: Concepts and Applications. Journal of Atomic, Molecular, Condensate and Nano Physics 2018, 5, 159-193, 10.26713/jamcnp.v5i2.842.
- Richard Balint; Nigel J. Cassidy; Sarah H. Cartmell; Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomaterialia 2014, 10, 2341-2353, 10.1016/j.actbio.2014.02.015.
- Thanh-Hai Le; Yukyung Kim; Hyeonseok Yoon; Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150, 10.3390/polym9040150.
- Mihaela Tertis; Anca Florea; Robert Săndulescu; Cecilia Cristea; Carbon Based Electrodes Modified with Horseradish Peroxidase Immobilized in Conducting Polymers for Acetaminophen Analysis. Sensors 2013, 13, 4841-4854, 10.3390/s130404841.
- Huseyin Bekir Yildiz; Salim Caliskan; Musa Kamaci; Abdullah Caliskan; Hasim Yilmaz; l-Dopa synthesis catalyzed by tyrosinase immobilized in poly(ethyleneoxide) conducting polymers. International Journal of Biological Macromolecules 2013, 56, 34-40, 10.1016/j.ijbiomac.2013.01.031.
- Jugović, B.; Grgur, B.; Antov, M.; Knežević-Jugović, Z.; Stevanović, J.; Gvozdenović, M.; Polypyrrole-based Enzyme Electrode with Immobilized Glucose Oxidase for Electrochemical Determination of Glucose. Int. J. Electrochem. Sci 2016, 11, 1152-1161.
- Taniya M. S. K. Pathiranage; Dushanthi S. Dissanayake; Crystal N. Niermann; Yixin Ren; Michael C. Biewer; Mihaela C. Stefan; Role of polythiophenes as electroactive materials. Journal of Polymer Science Part A: Polymer Chemistry 2017, 55, 3327-3346, 10.1002/pola.28726.
- L. Groenendaal; G. Zotti; P.-H. Aubert; S.M. Waybright; J.R. Reynolds; Electrochemistry of Poly(3,4-alkylenedioxythiophene) Derivatives. Advanced Materials 2003, 15, 855-879, 10.1002/adma.200300376.
- C. Zanardi; Fabio Terzi; R. Seeber; Polythiophenes and polythiophene-based composites in amperometric sensing. Analytical and Bioanalytical Chemistry 2012, 405, 509-531, 10.1007/s00216-012-6318-7.
- Guangyuan Xu; Zahraa A. Jarjes; Valentin Desprez; Paul Kilmartin; Jadranka Travas-Sejdic; Sensitive, selective, disposable electrochemical dopamine sensor based on PEDOT-modified laser scribed graphene. Biosensors and Bioelectronics 2018, 107, 184-191, 10.1016/j.bios.2018.02.031.
- Danfeng Sun; Hongji Li; Mingji Li; Cuiping Li; Hongli Dai; Dazhi Sun; Baohe Yang; Electrodeposition synthesis of a NiO/CNT/PEDOT composite for simultaneous detection of dopamine, serotonin, and tryptophan. Sensors and Actuators B: Chemical 2018, 259, 433-442, 10.1016/j.snb.2017.12.037.
- Juan José García Guzmán; Laura Cubillana Aguilera; Dolores Bellido Milla; Ignacio Naranjo Rodríguez; Cecilia Lete; Jose María Palacios Santander; Stelian Lupu; Development of Sonogel-Carbon based biosensors using sinusoidal voltages and currents methods. Sensors and Actuators B: Chemical 2018, 255, 1525-1535, 10.1016/j.snb.2017.08.161.
- Stelian Lupu; Cecilia Lete; Paul Catalin Balaure; Dan Ion Caval; Constantin Mihailciuc; Boris Lakard; Jean-Yves Hihn; Francisco Javier Del Campo; Development of Amperometric Biosensors Based on Nanostructured Tyrosinase-Conducting Polymer Composite Electrodes. Sensors 2013, 13, 6759-6774, 10.3390/s130506759.
- Merih Zeynep Çetin; Pinar Camurlu; An amperometric glucose biosensor based on PEDOT nanofibers. RSC Advances 2018, 8, 19724-19731, 10.1039/c8ra01385c.
- Mahnoush Beygisangchin; Suraya Abdul Rashid; Suhaidi Shafie; Amir Sadrolhosseini; Hong Lim; Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003, 10.3390/polym13122003.
- Hairui Zhang; Jixiao Wang; Xingbin Gao; Zhi Wang; Shichang Wang; The electrochemical activity of polyaniline: An important issue on its use in electrochemical energy storage devices. Synthetic Metals 2014, 187, 46-51, 10.1016/j.synthmet.2013.10.022.
- Rajesh Kashyap; Ravi Kumar; Mukesh Kumar; Sachin Tyagi; Dinesh Kumar; Polyaniline nanofibers based gas sensor for detection of volatile organic compounds at room temperature. Materials Research Express 2019, 6, 1150d3, 10.1088/2053-1591/ab4e43.
- Fern M. Kelly; Ludivine Meunier; Cédric Cochrane; Vladan Koncar; Polyaniline: Application as solid state electrochromic in a flexible textile display. Displays 2013, 34, 1-7, 10.1016/j.displa.2012.10.001.
- Zondi Nate; Atal A.S. Gill; Ruchika Chauhan; Rajshekhar Karpoormath; Polyaniline-cobalt oxide nanofibers for simultaneous electrochemical determination of antimalarial drugs: Primaquine and proguanil. Microchemical Journal 2020, 160, 105709, 10.1016/j.microc.2020.105709.
- Xiaoyu Zhao; Wenlong Bai; Yujia Yan; Yanfei Wang; Juankun Zhang; Core-Shell Self-Doped Polyaniline Coated Metal-Organic-Framework (SPAN@UIO-66-NH2) Screen Printed Electrochemical Sensor for Cd2+ Ions. Journal of The Electrochemical Society 2019, 166, B873-B880, 10.1149/2.0251912jes.
- C. Sanchis; M.A. Ghanem; H.J. Salavagione; E. Morallón; P.N. Bartlett; The oxidation of ascorbate at copolymeric sulfonated poly(aniline) coated on glassy carbon electrodes. Bioelectrochemistry 2011, 80, 105-113, 10.1016/j.bioelechem.2010.06.006.
- Fatemeh Masdarolomoor; Somayeh Hajizadeh; Mansoor Arab Chamjangali; Peter C. Innis; Novel approach to the synthesis of polyaniline possessing electroactivity at neutral pH. Synthetic Metals 2019, 250, 121-130, 10.1016/j.synthmet.2019.03.011.
- Habib Ullah; Anwar-Ul-Haq Ali Shah; Salma Bilal; Khurshid Ayub; Doping and Dedoping Processes of Polypyrrole: DFT Study with Hybrid Functionals. The Journal of Physical Chemistry C 2014, 118, 17819-17830, 10.1021/jp505626d.
- Pinar Camurlu; Polypyrrole derivatives for electrochromic applications. RSC Advances 2014, 4, 55832-55845, 10.1039/c4ra11827h.
- A. Ramanavičius; A. Ramanavičienė; A. Malinauskas; Electrochemical sensors based on conducting polymer—polypyrrole. Electrochimica Acta 2006, 51, 6025-6037, 10.1016/j.electacta.2005.11.052.
- Siddhant Jain; Mohan Singh Mehata; Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Scientific Reports 2017, 7, 1-13, 10.1038/s41598-017-15724-8.
- Krzysztof Maksymiuk; Chemical Reactivity of Polypyrrole and Its Relevance to Polypyrrole Based Electrochemical Sensors. Electroanalysis 2006, 18, 1537-1551, 10.1002/elan.200603573.
- Servet Cete; Munise Ozyurt; Ertan Yildirim; Deniz Akin; A novel biosensor with the use of polypyrrole–poly(sodium-4-styrenesulphonate) as a dopant in the determination of glucose. Chemical Papers 2019, 74, 799-808, 10.1007/s11696-019-00907-6.
- Joseph G. Ayenimo; Samuel B. Adeloju; Amperometric detection of glucose in fruit juices with polypyrrole-based biosensor with an integrated permselective layer for exclusion of interferences. Food Chemistry 2017, 229, 127-135, 10.1016/j.foodchem.2017.01.138.
- Lara F. Loguercio; Anderson Thesing; Pedro Demingos; Carlos D.L. de Albuquerque; Roberta S.B. Rodrigues; Alexandre G. Brolo; Jacqueline F.L. Santos; Efficient acetylcholinesterase immobilization for improved electrochemical performance in polypyrrole nanocomposite-based biosensors for carbaryl pesticide. Sensors and Actuators B: Chemical 2021, 339, 129875, 10.1016/j.snb.2021.129875.
- Tais Carpintero Barroso de Morais; Dayvison Ribeiro Rodrigues; Urijatan Teixeira De Carvalho Polari Souto; Sherlan G. Lemos; A simple voltammetric electronic tongue for the analysis of coffee adulterations. Food Chemistry 2019, 273, 31-38, 10.1016/j.foodchem.2018.04.136.
- Marta Podrażka; Ewa Bączyńska; Magdalena Kundys; Paulina S. Jeleń; Emilia Witkowska Nery; Electronic Tongue—A Tool for All Tastes?. Biosensors 2017, 8, 3, 10.3390/bios8010003.
- Lara Sobrino-Gregorio; Román Bataller; Juan Soto; Isabel Escriche; Monitoring honey adulteration with sugar syrups using an automatic pulse voltammetric electronic tongue. Food Control 2018, 91, 254-260, 10.1016/j.foodcont.2018.04.003.
- María José Aliaño-González; Marta Ferreiro-González; Gerardo F. Barbero; Jesús Ayuso; José A. Álvarez; Miguel Palma; Carmelo G. Barroso; An Electronic Nose Based Method for the Discrimination of Weathered Petroleum-Derived Products. Sensors 2018, 18, 2180, 10.3390/s18072180.
- Maimunah Mohd Ali; Norhashila Hashim; Samsuzana Abd Aziz; Ola Lasekan; Principles and recent advances in electronic nose for quality inspection of agricultural and food products. Trends in Food Science & Technology 2020, 99, 1-10, 10.1016/j.tifs.2020.02.028.
- Limin Lu; Electrochemical Sensor Based on poly(3,4-ethylenedioxy - thiophene) Doped with Transition Metals for Detecting Rutin in Buck Wheat Tea. International Journal of Electrochemical Science 2018, 13, 2126-2135, 10.20964/2018.02.66.
- Jorge G. Ibanez; Marina. E. Rincón; Silvia Gutierrez-Granados; M’Hamed Chahma; Oscar Andrés Jaramillo-Quintero; Bernardo A. Frontana-Uribe; Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical–Chiral Sensors. Chemical Reviews 2018, 118, 4731-4816, 10.1021/acs.chemrev.7b00482.
- V.S. Vasantha; Shen-Ming Chen; Electrocatalysis and simultaneous detection of dopamine and ascorbic acid using poly(3,4-ethylenedioxy)thiophene film modified electrodes. Journal of Electroanalytical Chemistry 2006, 592, 77-87, 10.1016/j.jelechem.2006.04.026.
- S. Brillians Revin; S. Abraham John; Simultaneous determination of two important dopamine metabolites at physiological pH by voltammetry. Analytical Methods 2012, 4, 348-352, 10.1039/c2ay05664j.
- S. Brillians Revin; Abraham John; Electrochemical sensor for neurotransmitters at physiological pH using a heterocyclic conducting polymer modified electrode. The Analyst 2011, 137, 209-215, 10.1039/c1an15746a.
- Limin Yang; Shufeng Liu; Qixiu Zhang; Feng Li; Simultaneous electrochemical determination of dopamine and ascorbic acid using AuNPs@polyaniline core–shell nanocomposites modified electrode. Talanta 2012, 89, 136-141, 10.1016/j.talanta.2011.12.002.
- Protiva Rani Roy; Takeyoshi Okajima; Takeo Ohsaka; Simultaneous electroanalysis of dopamine and ascorbic acid using poly (N,N-dimethylaniline)-modified electrodes. Bioelectrochemistry 2002, 59, 11-19, 10.1016/s1567-5394(02)00156-1.
- Jun Wan; Yuxiao Si; Chao Li; Kun Zhang; Bisphenol a electrochemical sensor based on multi-walled carbon nanotubes/polythiophene/Pt nanocomposites modified electrode. Analytical Methods 2016, 8, 3333-3338, 10.1039/C6AY00850J.
- T. Ramachandran; V. Violet Dhayabaran; Utilization of a MnO2/polythiophene/rGO nanocomposite modified glassy carbon electrode as an electrochemical sensor for methyl parathion. Journal of Materials Science: Materials in Electronics 2019, 30, 12315-12327, 10.1007/s10854-019-01590-9.
- Asma Saljooqi; Tayebeh Shamspur; Ali Mostafavi; The electrochemical sensor based on graphene oxide nanosheets decorated by gold nanoparticles and polythiophene for nicotine sensing in biological samples and cigarette. Journal of Materials Science: Materials in Electronics 2020, 31, 5471-5477, 10.1007/s10854-020-03111-5.
- Seyyed Alireza Hashemi; Seyyed Mojtaba Mousavi; Sonia Bahrani; Seeram Ramakrishna; Polythiophene silver bromide nanostructure as ultra-sensitive non-enzymatic electrochemical glucose biosensor. European Polymer Journal 2020, 138, 109959, 10.1016/j.eurpolymj.2020.109959.
- S. Dheepthi GunaVathana; P. Thivya; J. Wilson; A. Cyrac Peter; Sensitive voltammetric sensor based on silver dendrites decorated polythiophene nanocomposite: Selective determination of L-Tryptophan. Journal of Molecular Structure 2020, 1205, 109959, 10.1016/j.molstruc.2019.127649.
- Zhen Song; Ge Sheng; Yige Cui; Mengru Li; Zhiling Song; Caifeng Ding; Xiliang Luo; Low fouling electrochemical sensing in complex biological media by using the ionic liquid-doped conducting polymer PEDOT: application to voltammetric determination of dopamine. Microchimica Acta 2019, 186, 220, 10.1007/s00604-019-3340-x.
- Qingyun Tian; Jingkun Xu; Yinxiu Zuo; Yingying Li; Jialing Zhang; Yaoyi Zhou; Xuemin Duan; Limin Lu; Haiyan Jia; Quan Xu; et al.Yongfang Yu Three-dimensional PEDOT composite based electrochemical sensor for sensitive detection of chlorophenol. Journal of Electroanalytical Chemistry 2019, 837, 1-9, 10.1016/j.jelechem.2019.01.055.
- Mengru Li; Wei Wang; Zhuo Chen; Zhiling Song; Xiliang Luo; Electrochemical determination of paracetamol based on Au@graphene core-shell nanoparticles doped conducting polymer PEDOT nanocomposite. Sensors and Actuators B: Chemical 2018, 260, 778-785, 10.1016/j.snb.2018.01.093.
- Li-Na Wu; Jing-Ping Zhong; Muhammad Waqas; Zhe Jiang; You-Jun Fan; Yue Sun; Jia Li; Wei Chen; Controllable synthesis of six corner star-like Cu2O/PEDOT-MWCNT composites and their performance toward electrochemical glucose sensing. Electrochimica Acta 2019, 318, 837-846, 10.1016/j.electacta.2019.06.124.
- Davide Bottari; Laura Pigani; Chiara Zanardi; Fabio Terzi; Sanda Victorinne Paţurcă; Sorin Dan Grigorescu; Cristian Matei; Cecilia Lete; Stelian Lupu; Electrochemical Sensing of Caffeic Acid Using Gold Nanoparticles Embedded in Poly(3,4-ethylenedioxythiophene) Layer by Sinusoidal Voltage Procedure. Chemosensors 2019, 7, 65, 10.3390/chemosensors7040065.
- Juan José García-Guzmán; David López-Iglesias; Laura Cubillana-Aguilera; Cecilia Lete; Stelian Lupu; José María Palacios-Santander; Dolores Bellido Milla; Assessment of the Polyphenol Indices and Antioxidant Capacity for Beers and Wines Using a Tyrosinase-Based Biosensor Prepared by Sinusoidal Current Method. Sensors 2018, 19, 66, 10.3390/s19010066.
- Yi Ge; Ruxangul Jamal; Ruanye Zhang; Wenli Zhang; Zongna Yu; Yinqiang Yan; Yingcheng Liu; Tursun Abdiryim; Electrochemical synthesis of multilayered PEDOT/PEDOT-SH/Au nanocomposites for electrochemical sensing of nitrite.. Microchimica Acta 2020, 187, 248-10, 10.1007/s00604-020-4211-1.
- Mahsa Motshakeri; Anthony R. J. Phillips; Jadranka Travas‐Sejdic; Paul A. Kilmartin; Electrochemical Study of Gold Microelectrodes Modified with PEDOT to Quantify Uric Acid in Milk Samples. Electroanalysis 2020, 32, 2101-2111, 10.1002/elan.202060086.
- Bowen Zhang; Abdelhadi El Jaouhari; Xiangrong Wu; Wei Liu; Jinhua Zhu; Xiuhua Liu; Synthesis and characterization of PEDOT-MC decorated AgNPs for voltammetric detection of rutin in real samples. Journal of Electroanalytical Chemistry 2020, 877, 114632, 10.1016/j.jelechem.2020.114632.
- Cecilia Lete; Mariana Marin; Elena Maria Anghel; Loredana Preda; Cristian Matei; Stelian Lupu; Sinusoidal voltage electrodeposition of PEDOT-Prussian blue nanoparticles composite and its application to amperometric sensing of H2O2 in human blood. Materials Science and Engineering: C 2019, 102, 661-669, 10.1016/j.msec.2019.04.086.
- Mohamed A. Yassin; Bishnu Kumar Shrestha; Rafiq Ahmad; Sita Shrestha; Chan Hee Park; Cheol Sang Kim; Exfoliated nanosheets of Co3O4 webbed with polyaniline nanofibers: A novel composite electrode material for enzymeless glucose sensing application. Journal of Industrial and Engineering Chemistry 2019, 73, 106-117, 10.1016/j.jiec.2019.01.011.
- Elhameh Saeb; Karim Asadpour- Zeynali; [Email Protected]; Facile synthesis of TiO2@PANI@Au nanocomposite as an electrochemical sensor for determination of hydrazine. Microchemical Journal 2020, 160, 105603, 10.1016/j.microc.2020.105603.
- Chengqian Duan; Jianbin Zheng; Porous coralloid Polyaniline/SnO2-based enzyme-free sensor for sensitive and selective detection of nitrite. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2019, 567, 271-277, 10.1016/j.colsurfa.2019.01.063.
- Omolola E. Fayemi; Abolanle Adekunle; B.E.Kumara Swamy; Eno E. Ebenso; Electrochemical sensor for the detection of dopamine in real samples using polyaniline/NiO, ZnO, and Fe3O4 nanocomposites on glassy carbon electrode. Journal of Electroanalytical Chemistry 2018, 818, 236-249, 10.1016/j.jelechem.2018.02.027.
- M.R. Mahmoudian; W.J. Basirun; M. Sookhakian; Pei Meng Woi; E. Zalnezhad; H. Hazarkhani; Y. Alias; Synthesis and characterization of α-Fe2O3/polyaniline nanotube composite as electrochemical sensor for uric acid detection. Advanced Powder Technology 2018, 30, 384-392, 10.1016/j.apt.2018.11.015.
- Saraswathi Kailasaa; B. Geeta Rani; M. Sai Bhargava Reddy; N. Jayarambabu; P. Munindra; Sanjeev Sharma; K. Venkateswara Rao; NiO nanoparticles -decorated conductive polyaniline nanosheets for amperometric glucose biosensor. Materials Chemistry and Physics 2019, 242, 122524, 10.1016/j.matchemphys.2019.122524.
- Seyed Morteza Naghib; Farahnaz Behzad; Mehdi Rahmanian; Yasser Zare; Kyong Yop Rhee; A highly sensitive biosensor based on methacrylated graphene oxide-grafted polyaniline for ascorbic acid determination. Nanotechnology Reviews 2020, 9, 760-767, 10.1515/ntrev-2020-0061.
- Azadeh Tavousi; Elahe Ahmadi; Leila Mohammadi-Behzad; Vahid Riahifar; Fatemeh Maghemi; Sensitive electrochemical sensor using polypyrrole-coated Fe3O4 core-shell nanoparticles/multiwall carbon nanotubes modified graphite electrode for atorvastatin analysis. Microchemical Journal 2020, 158, 105159, 10.1016/j.microc.2020.105159.
- Ayyadurai Kannan; Sivaprakasam Radhakrishnan; Fabrication of an electrochemical sensor based on gold nanoparticles functionalized polypyrrole nanotubes for the highly sensitive detection of l-dopa. Materials Today Communications 2020, 25, 101330, 10.1016/j.mtcomm.2020.101330.
- Waheed A. Adeosun; Abdullah M. Asiri; Hadi M. Marwani; Mohammed M. Rahman; Enzymeless Electrocatalytic Detection of Uric Acid Using Polydopamine/Polypyrrole Copolymeric film. ChemistrySelect 2020, 5, 156-164, 10.1002/slct.201903628.
- Vahid Arabali; Samira Malekmohammadi; Fatemeh Karimi; Surface amplification of pencil graphite electrode using CuO nanoparticle/polypyrrole nanocomposite; a powerful electrochemical strategy for determination of tramadol. Microchemical Journal 2020, 158, 105179, 10.1016/j.microc.2020.105179.
- Priscilla Mengarda; Fernando Augusto Lavezzo Dias; João Victor Peixoto; Raul Osiecki; Márcio F. Bergamini; Luiz H. Marcolino-Junior; Determination of lactate levels in biological fluids using a disposable ion-selective potentiometric sensor based on polypyrrole films. Sensors and Actuators B: Chemical 2019, 296, 126663, 10.1016/j.snb.2019.126663.
- Junping Ma; Jianbin Zheng; Voltammetric determination of hydrogen peroxide using AuCu nanoparticles attached on polypyrrole-modified 2D metal-organic framework nanosheets. Microchimica Acta 2020, 187, 1-8, 10.1007/s00604-020-04355-y.
- Da Ha; Qiyong Sun; Kaiqi Su; Hao Wan; Haibo Li; Ning Xu; Fei Sun; Liujing Zhuang; Ning Hu; Ping Wang; et al. Recent achievements in electronic tongue and bioelectronic tongue as taste sensors. Sensors and Actuators B: Chemical 2014, 207, 1136-1146, 10.1016/j.snb.2014.09.077.
- Manel del Valle; Electronic Tongues Employing Electrochemical Sensors. Electroanalysis 2010, 22, 1539-1555, 10.1002/elan.201000013.
- Clara Pérez-Ràfols; Núria Serrano; Cristina Ariño; Miquel Esteban; José Manuel Díaz-Cruz; Pérez- Ràfols; Díaz- Cruz; Voltammetric Electronic Tongues in Food Analysis. Sensors 2019, 19, 4261, 10.3390/s19194261.
- Dmitry Kirsanov; Daniel S. Correa; Gabriel Gaal; Antonio Riul; Maria L. Braunger; Flavio M. Shimizu; Osvaldo N. Oliveira; Tao Liang; Hao Wan; Ping Wang; et al.Ekaterina OlenevaAndrey Legin Electronic Tongues for Inedible Media. Sensors 2019, 19, 5113, 10.3390/s19235113.
- A. Riul; R.R. Malmegrim; F.J. Fonseca; L.H.C. Mattoso; An artificial taste sensor based on conducting polymers. Biosensors and Bioelectronics 2003, 18, 1365-1369, 10.1016/s0956-5663(03)00069-1.
- Jr. A. Riul; Jr. D. S. dos Santos; ‡ K. Wohnrath; ‡ R. Di Tommazo; § A. C. P. L. F. Carvalho; ‖ F. J. Fonseca; Jr. O. N. Oliveira; ⊥ And D. M. Taylor; † L. H. C. Mattoso; Artificial Taste Sensor: Efficient Combination of Sensors Made from Langmuir−Blodgett Films of Conducting Polymers and a Ruthenium Complex and Self-Assembled Films of an Azobenzene-Containing Polymer. Langmuir 2001, 18, 239-245, 10.1021/la011017d.
- A. Riul; A.M. Gallardo Soto; S.V. Mello; S. Bone; D.M. Taylor; L.H.C. Mattoso; An electronic tongue using polypyrrole and polyaniline. Synthetic Metals 2003, 132, 109-116, 10.1016/s0379-6779(02)00107-8.
- A.A Arrieta; C Apetrei; Maria Luz Rodriguez-Mendez; J.A de Saja; Voltammetric sensor array based on conducting polymer-modified electrodes for the discrimination of liquids. Electrochimica Acta 2004, 49, 4543-4551, 10.1016/j.electacta.2004.05.010.
- Larisa Lvova; Andrey Legin; Yuri Vlasov; Geun Sig Cha; Hakhyun Nam; Multicomponent analysis of Korean green tea by means of disposable all-solid-state potentiometric electronic tongue microsystem. Sensors and Actuators B: Chemical 2003, 95, 391-399, 10.1016/s0925-4005(03)00445-3.
- L. Pigani; G. Vasile Simone; G. Foca; A. Ulrici; F. Masino; Laura Cubillana Aguilera; Rosalba Calvini; R. Seeber; Prediction of parameters related to grape ripening by multivariate calibration of voltammetric signals acquired by an electronic tongue. Talanta 2018, 178, 178-187, 10.1016/j.talanta.2017.09.027.
- L. Pigani; A. Culetu; A. Ulrici; G. Foca; M. Vignali; R. Seeber; Pedot modified electrodes in amperometric sensing for analysis of red wine samples. Food Chemistry 2011, 129, 226-233, 10.1016/j.foodchem.2011.04.046.
- V. Martina; K. Ionescu; L. Pigani; F. Terzi; A. Ulrici; C. Zanardi; R. Seeber; Development of an electronic tongue based on a PEDOT-modified voltammetric sensor. Analytical and Bioanalytical Chemistry 2007, 387, 2101-2110, 10.1007/s00216-006-1102-1.
- Vanessa P. Scagion; Luiza A. Mercante; Karine Y. Sakamoto; Juliano E. Oliveira; Fernando J. Fonseca; Luiz H. C. Mattoso; Marcos D. Ferreira; Daniel S. Correa; An electronic tongue based on conducting electrospun nanofibers for detecting tetracycline in milk samples. RSC Advances 2016, 6, 103740-103746, 10.1039/c6ra21326j.
- Yongchao Yu; Pooran C. Joshi; Jayne Wu; Anming Hu; Laser-Induced Carbon-Based Smart Flexible Sensor Array for Multiflavors Detection. ACS Applied Materials & Interfaces 2018, 10, 34005-34012, 10.1021/acsami.8b12626.
- C. Garcia-Hernandez; C. Salvo-Comino; F. Martin-Pedrosa; C. Garcia-Cabezon; M.L. Rodriguez-Mendez; Analysis of red wines using an electronic tongue and infrared spectroscopy. Correlations with phenolic content and color parameters. LWT 2019, 118, 108785, 10.1016/j.lwt.2019.108785.
- Elisabeta-Irina Geană; Corina Teodora Ciucure; Victoria Artem; Constantin Apetrei; Wine varietal discrimination and classification using a voltammetric sensor array based on modified screen-printed electrodes in conjunction with chemometric analysis. Microchemical Journal 2020, 159, 105451, 10.1016/j.microc.2020.105451.
- Vicente Parra; Álvaro A. Arrieta; José-A. Fernández-Escudero; María Luz Rodríguez-Méndez; José Antonio De Saja; Electronic tongue based on chemically modified electrodes and voltammetry for the detection of adulterations in wines. Sensors and Actuators B: Chemical 2006, 118, 448-453, 10.1016/j.snb.2006.04.043.
- Irina Mirela Apetrei; C. Apetrei; Application of voltammetric e-tongue for the detection of ammonia and putrescine in beef products. Sensors and Actuators B: Chemical 2016, 234, 371-379, 10.1016/j.snb.2016.05.005.
- Álvaro A. Arrieta; Maria Luz Rodriguez-Mendez; José A. de Saja; Carlos A. Blanco; Dieudonné Nimubona; Prediction of bitterness and alcoholic strength in beer using an electronic tongue. Food Chemistry 2010, 123, 642-646, 10.1016/j.foodchem.2010.05.006.
- Constantin Apetrei; Novel method based on polypyrrole‐modified sensors and emulsions for the evaluation of bitterness in extra virgin olive oils. Food Research International 2012, 48, 673-680, 10.1016/j.foodres.2012.06.010.
- Elisabeta-Irina Geană; Victoria Artem; Constantin Apetrei; Discrimination and classification of wines based on polypyrrole modified screen-printed carbon electrodes coupled with multivariate data analysis. Journal of Food Composition and Analysis 2020, 96, 103704, 10.1016/j.jfca.2020.103704.
- Juan Manuel Gutiérrez; Laura Moreno-Barón; Maria Isabel Pividori; Salvador Alegret; Manel del Valle; A voltammetric electronic tongue made of modified epoxy-graphite electrodes for the qualitative analysis of wine. Microchimica Acta 2010, 169, 261-268, 10.1007/s00604-010-0351-z.
- K. Arshak; E. Moore; G.M. Lyons; J. Harris; Seamus Clifford; A review of gas sensors employed in electronic nose applications. Sensor Review 2004, 24, 181-198, 10.1108/02602280410525977.
- Pirsa, S.. Materials Science and Engineering: Concepts, Methodologies, Tools, and Applications; IGI Global: USA, 2017; pp. 150-180.
- Boris Lakard; Stéphanie Carquigny; Olivier Segut; Tilia Patois; Sophie Lakard; Gas Sensors Based on Electrodeposited Polymers. Metals 2015, 5, 1371-1386, 10.3390/met5031371.
- Seon Joo Park; Chul Soon Park; Hyeonseok Yoon; Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids. Polymers 2017, 9, 155, 10.3390/polym9050155.
- Weiwei Wu; Bin Wang; Meital Segev-Bar; Wei Dou; Fang Niu; Yehu David Horev; Yunfeng Deng; Marian Plotkin; Tan-Phat Huynh; Raneen Jeries; et al.Han ZhuA'laa GaraaShifaa' BadarnehLinfeng ChenMingliang DuWenwen HuHossam Haick Free-Standing and Eco-Friendly Polyaniline Thin Films for Multifunctional Sensing of Physical and Chemical Stimuli. Advanced Functional Materials 2017, 27, 1703147, 10.1002/adfm.201703147.
- Tao Zhang; Haoyuan Qi; Zhongquan Liao; Yehu David Horev; Luis Antonio Panes-Ruiz; Petko St. Petkov; Zhe Zhang; Rishi Shivhare; Panpan Zhang; Kejun Liu; et al.Viktor BezuglyShaohua LiuZhikun ZhengStefan MannsfeldThomas HeineGianaurelio CunibertiHossam HaickEhrenfried ZschechUte KaiserRenhao DongXinliang Feng Engineering crystalline quasi-two-dimensional polyaniline thin film with enhanced electrical and chemiresistive sensing performances. Nature Communications 2019, 10, 1-9, 10.1038/s41467-019-11921-3.
- Caroline Duc; Mohamed-Lamine Boukhenane; Jean-Luc Wojkiewicz; Nathalie Redon; Hydrogen Sulfide Detection by Sensors Based on Conductive Polymers: A Review. Frontiers in Materials 2020, 7, 215, 10.3389/fmats.2020.00215.
- S. Mikhaylov; N. A. Ogurtsov; N. Redon; P. Coddeville; J.-L. Wojkiewicz; A. A. Pud; The PANI-DBSA content and dispersing solvent as influencing parameters in sensing performances of TiO2/PANI-DBSA hybrid nanocomposites to ammonia. RSC Advances 2016, 6, 82625-82634, 10.1039/c6ra12693f.
- Kyung Hwa Hong; Kyung Wha Oh; Tae Jin Kang; Polyaniline-nylon 6 composite fabric for ammonia gas sensor. Journal of Applied Polymer Science 2004, 92, 37-42, 10.1002/app.13633.
- H Hu; M Trejo; M.E Nicho; J.M Saniger; A Garcı́a-Valenzuela; Adsorption kinetics of optochemical NH3 gas sensing with semiconductor polyaniline films. Sensors and Actuators B: Chemical 2002, 82, 14-23, 10.1016/s0925-4005(01)00984-4.
- Mijuan Xu; Jun Zhang; Shurong Wang; Xianzhi Guo; Huijuan Xia; Yan Wang; Shoumin Zhang; Weiping Huang; Shihua Wu; Gas sensing properties of SnO2 hollow spheres/polythiophene inorganic–organic hybrids. Sensors and Actuators B: Chemical 2010, 146, 8-13, 10.1016/j.snb.2010.01.053.
- YaQiong Zhang; Benjamin R. Bunes; Na Wu; Adam Ansari; Saleha Rajabali; Ling Zang; Sensing methamphetamine with chemiresistive sensors based on polythiophene-blended single-walled carbon nanotubes. Sensors and Actuators B: Chemical 2018, 255, 1814-1818, 10.1016/j.snb.2017.08.201.
- Dawu Lv; Weigang Chen; Wenfeng Shen; Mingyue Peng; Xuesong Zhang; Runfei Wang; Lei Xu; Wei Xu; Weijie Song; Ruiqin Tan; et al. Enhanced flexible room temperature ammonia sensor based on PEDOT: PSS thin film with FeCl3 additives prepared by inkjet printing. Sensors and Actuators B: Chemical 2019, 298, 298, 10.1016/j.snb.2019.126890.
- Jagjeevan Ram; R. G. Singh; Fouran Singh; Vikas Kumar; Vishnu Chauhan; Rashi Gupta; Utkarsh Kumar; B. C. Yadav; Rajesh Kumar; Development of WO3-PEDOT: PSS hybrid nanocomposites based devices for liquefied petroleum gas (LPG) sensor. Journal of Materials Science: Materials in Electronics 2019, 30, 13593-13603, 10.1007/s10854-019-01728-9.
- Pengyu Yang; Dawu Lv; Wenfeng Shen; Tieyi Wu; Ye Yang; Yue Zhao; Ruiqin Tan; Weijie Song; Porous flexible polyaniline/polyvinylidene fluoride composite film for trace-level NH3 detection at room temperature. Materials Letters 2020, 271, 127798, 10.1016/j.matlet.2020.127798.
- Hongyan Xu; Dianxing Ju; Wenru Li; Haibo Gong; Jun Zhang; Jieqiang Wang; Bingqiang Cao; Low-working-temperature, fast-response-speed NO2 sensor with nanoporous-SnO2/polyaniline double-layered film. Sensors and Actuators B: Chemical 2016, 224, 654-660, 10.1016/j.snb.2015.10.076.
- Dongzhi Zhang; Zhenling Wu; Xiaoqi Zong; Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sensors and Actuators B: Chemical 2019, 289, 32-41, 10.1016/j.snb.2019.03.055.
- S. Kotresh; Y. T. Ravikiran; S. C. Vijayakumari; Sabu Thomas; Interfacial p-n heterojunction of polyaniline-nickel ferrite nanocomposite as room temperature liquefied petroleum gas sensor. Composite Interfaces 2016, 24, 549-561, 10.1080/09276440.2017.1241523.
- Xiaohui Tang; Jean-Pierre Raskin; Nadzeya Kryvutsa; Sophie Hermans; Oleksandr Slobodian; Alexei N. Nazarov; Marc Debliquy; An ammonia sensor composed of polypyrrole synthesized on reduced graphene oxide by electropolymerization. Sensors and Actuators B: Chemical 2019, 305, 127423, 10.1016/j.snb.2019.127423.
- Sachin Navale; A.T. Mane; M.A. Chougule; R.D. Sakhare; S.R. Nalage; V.B. Patil; Highly selective and sensitive room temperature NO2 gas sensor based on polypyrrole thin films. Synthetic Metals 2014, 189, 94-99, 10.1016/j.synthmet.2014.01.002.
- Arpita Adhikari; Punam Tiwary; Dipak Rana; Arijit Halder; Jyotisko Nath; Arijita Basu; Debojyoti Ghoshal; Pradip Kar; Amit Kumar Chakraborty; Dipankar Chattopadhyay; et al. Na-cholate micelle mediated synthesis of polypyrrole nanoribbons for ethanol sensing. Journal of Environmental Chemical Engineering 2020, 8, 104249, 10.1016/j.jece.2020.104249.
- Arpita Adhikari; Pradip Kar; Dipak Rana; Sriparna De; Jyotishka Nath; Kingshuk Dutta; Dipankar Chattopadhyay; Synthesis of sodium cholate mediated rod-like polypyrrole-silver nanocomposite for selective sensing of acetone vapor. Nano-Structures & Nano-Objects 2020, 21, 100419, 10.1016/j.nanoso.2019.100419.
- Seon Joo Park; Oh Seok Kwon; Jyongsik Jang; A high-performance hydrogen gas sensor using ultrathin polypyrrole-coated CNT nanohybrids. Chemical Communications 2013, 49, 4673-4675, 10.1039/c3cc41020j.
- Bhagaban Behera; Rathin Joshi; G K Anil Vishnu; Sanjay Bhalerao; Hardik J Pandya; Electronic nose: a non-invasive technology for breath analysis of diabetes and lung cancer patients. Journal of Breath Research 2019, 13, 024001, 10.1088/1752-7163/aafc77.
- Alphus Dan Wilson; Application of Electronic-Nose Technologies and VOC-Biomarkers for the Noninvasive Early Diagnosis of Gastrointestinal Diseases. Sensors 2018, 18, 2613, 10.3390/s18082613.
- Baishu Liu; Yan Huang; Kenneth Wl. Kam; Wai-Fung Cheung; Ni Zhao; Bo Zheng; Functionalized graphene-based chemiresistive electronic nose for discrimination of disease-related volatile organic compounds. Biosensors and Bioelectronics: X 2019, 1, 100016, 10.1016/j.biosx.2019.100016.
- Nahid Aghili Nategh; Mohammad Jafar Dalvand; Adieh Anvar; Detection of toxic and non-toxic sweet cherries at different degrees of maturity using an electronic nose. Journal of Food Measurement and Characterization 2020, 15, 1213-1224, 10.1007/s11694-020-00724-6.
- J.V. Hatfield; P. Neaves; P.J. Hicks; Krishna Persaud; P. Travers; Towards an integrated electronic nose using conducting polymer sensors. Sensors and Actuators B: Chemical 1994, 18, 221-228, 10.1016/0925-4005(94)87086-1.
- Rita Stella; Joseph N Barisci; Giorgio Serra; Gordon Wallace; Danilo De Rossi; Characterisation of olive oil by an electronic nose based on conducting polymer sensors. Sensors and Actuators B: Chemical 2000, 63, 1-9, 10.1016/s0925-4005(99)00510-9.
- Joseph N. Barisci; Gordon G. Wallace; Michael K. Andrews; Ashton C. Partridge; Paul D. Harris; Conducting polymer sensors for monitoring aromatic hydrocarbons using an electronic nose. Sensors and Actuators B: Chemical 2002, 84, 252-257, 10.1016/s0925-4005(02)00033-3.
- Tanushree Sen; Satyendra Mishra; Navinchandra G. Shimpi; Synthesis and sensing applications of polyaniline nanocomposites: a review. RSC Advances 2016, 6, 42196-42222, 10.1039/c6ra03049a.
- Sadanand Pandey; Highly sensitive and selective chemiresistor gas/vapor sensors based on polyaniline nanocomposite: A comprehensive review. Journal of Science: Advanced Materials and Devices 2016, 1, 431-453, 10.1016/j.jsamd.2016.10.005.
- Haizhou Liu; Yunhui Wu; Song Chen; Shuqi Liu; Pingping Wang; Feng Xue; Lan Liu; A flexible and multifunctional electronic nose using polyaniline/cotton fibrous membrane with a hierarchical structure. Materials Letters 2018, 233, 324-327, 10.1016/j.matlet.2018.09.046.
- Lídia Tiggemann; Sandra Cristina Ballen; Cristian Matheus Bocalon; Adriana Marcia Graboski; Alexandra Manzoli; Juliana Steffens; Eunice Valduga; Clarice Steffens; Electronic nose system based on polyaniline films sensor array with different dopants for discrimination of artificial aromas. Innovative Food Science & Emerging Technologies 2017, 43, 112-116, 10.1016/j.ifset.2017.08.003.
- Adriana Marcia Graboski; Sandra Cristina Ballen; Elisiane Galvagni; Thiago Lazzari; Alexandra Manzoli; Flávio Makoto Shimizu; Juliana Steffens; Clarice Steffens; Aroma detection using a gas sensor array with different polyaniline films. Analytical Methods 2019, 11, 654-660, 10.1039/c8ay02389a.
- Alexandra Manzoli; Clarice Steffens; Rafaella Takehara Paschoalin; Adriana Marcia Graboski; Humberto De Mello Brandão; Bruno Campos de Carvalho; José Luiz Bellini; Paulo Sergio De Paula Herrmann; Volatile compounds monitoring as indicative of female cattle fertile period using electronic nose. Sensors and Actuators B: Chemical 2018, 282, 609-616, 10.1016/j.snb.2018.11.109.
- Alexandra Manzoli; Clarice Steffens; Rafaella Takehara Paschoalin; Alessandra A. Correa; William F. Alves; Fabio L. Leite; Paulo S. P. Herrmann; Low-Cost Gas Sensors Produced by the Graphite Line-Patterning Technique Applied to Monitoring Banana Ripeness. Sensors 2011, 11, 6425-6434, 10.3390/s110606425.
- Sandra Cristina Ballen; Adriana Marcia Graboski; Alexandra Manzoli; Juliana Steffens; Clarice Steffens; Monitoring Aroma Release in Gummy Candies During The Storage Using Electronic Nose. Food Analytical Methods 2019, 13, 3-12, 10.1007/s12161-019-01496-6.
- Paul Le Maout; Jean-Luc Wojkiewicz; Nathalie Redon; Cyril Lahuec; Fabrice Seguin; Laurent Dupont; Sergei Mikhaylov; Yuriy Noskov; Nikolay Ogurtsov; Alexander Pud; et al. Polyaniline nanocomposites based sensor array for breath ammonia analysis. Portable e-nose approach to non-invasive diagnosis of chronic kidney disease. Sensors and Actuators B: Chemical 2018, 274, 616-626, 10.1016/j.snb.2018.07.178.
- P. Le Maout; P.S Laquintinie; C. Lahuec; F. Seguin; J-L. Wojkiewicz; N. Redon; L. Dupont; A low cost, handheld E-nose for renal diseases early diagnosis. 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2018, 2018, 2817-2820, 10.1109/embc.2018.8512847.
- Adriana Marcia Graboski; Claudio A. Zakrzevski; Flavio Makoto Shimizu; Rafaella Takehara Paschoalin; Andrey Coatrini Soares; Juliana Steffens; Natalia Paroul; Clarice Steffens; Electronic Nose Based on Carbon Nanocomposite Sensors for Clove Essential Oil Detection. ACS Sensors 2020, 5, 1814-1821, 10.1021/acssensors.0c00636.
- Ambra R. Di Rosa; Anna M. F. Marino; Francesco Leone; Giuseppe G. Corpina; Renato P. Giunta; Vincenzo Chiofalo; Characterization of Sicilian Honeys Pollen Profiles Using a Commercial E-Tongue and Melissopalynological Analysis for Rapid Screening: A Pilot Study. Sensors 2018, 18, 4065, 10.3390/s18114065.
- Nikola Major; Ksenija Marković; Marina Krpan; Goran Šarić; Mirjana Hruškar; Nada Vahčić; Rapid honey characterization and botanical classification by an electronic tongue. Talanta 2011, 85, 569-574, 10.1016/j.talanta.2011.04.025.
- Xinzhuang Zhang; Yawei Zhang; Qingxiang Meng; Ning Li; Liping Ren; Evaluation of Beef by Electronic Tongue System TS-5000Z: Flavor Assessment, Recognition and Chemical Compositions According to Its Correlation with Flavor. PLoS ONE 2015, 10, e0137807, 10.1371/journal.pone.0137807.
- Katharina Woertz; Corinna Tissen; Peter Kleinebudde; Jörg Breitkreutz; A comparative study on two electronic tongues for pharmaceutical formulation development. Journal of Pharmaceutical and Biomedical Analysis 2011, 55, 272-281, 10.1016/j.jpba.2011.02.002.
- Miao Liu; Jun Wang; Duo Li; Mingjun Wang; Electronic Tongue Coupled with Physicochemical Analysis for the Recognition of Orange Beverages. Journal of Food Quality 2012, 35, 429-441, 10.1111/jfq.12004.
- Miriam Pein; Dmitry Kirsanov; Patrycja Ciosek; Manel del Valle; Irina Yaroshenko; Małgorzata Wesoły; Marcin Zabadaj; Andreu Gonzalez-Calabuig; Wojciech Wróblewski; Andrey Legin; et al. Independent comparison study of six different electronic tongues applied for pharmaceutical analysis. Journal of Pharmaceutical and Biomedical Analysis 2015, 114, 321-329, 10.1016/j.jpba.2015.05.026.
- Madara Tirzīte; Māris Bukovskis; Gunta Strazda; Normunds Jurka; Immanuels Taivans; Detection of lung cancer with electronic nose and logistic regression analysis. Journal of Breath Research 2018, 13, 016006, 10.1088/1752-7163/aae1b8.
- Or Herman-Saffar; Zvi Boger; Shai Libson; David Lieberman; Raphael Gonen; Yehuda Zeiri; Early non-invasive detection of breast cancer using exhaled breath and urine analysis. Computers in Biology and Medicine 2018, 96, 227-232, 10.1016/j.compbiomed.2018.04.002.
- Lorena Díaz De León-Martíne; Maribel Rodríguez-Aguilar; Patricia Gorocica-Rosete; Carlos Alberto Domínguez-Reyes; Verónica Martínez-Bustos; Juan Alberto Tenorio-Torres; Omar Ornelas-Rebolledo; José Alfonso Cruz-Ramos; Berenice Balderas-Segura; Rogelio Flores-Ramírez; et al. Identification of profiles of volatile organic compounds in exhaled breath by means of an electronic nose as a proposal for a screening method for breast cancer: a case-control study. Journal of Breath Research 2020, 14, 046009, 10.1088/1752-7163/aba83f.
- Mariana Farraia; João Cavaleiro Rufo; Inês Paciência; Francisca Castro Mendes; Ana Rodolfo; Tiago Azenha Rama; Sílvia Rocha; Luís Delgado; Paul Brinkman; André Moreira; et al. Human volatilome analysis using eNose to assess uncontrolled asthma in a clinical setting. Allergy 2020, 75, 1630-1639, 10.1111/all.14207.
- Laura Tenero; Marco Sandri; Michele Piazza; Giulia Paiola; Marco Zaffanello; Giorgio L Piacentini; Electronic nose in discrimination of children with uncontrolled asthma. Journal of Breath Research 2020, 14, 046003, 10.1088/1752-7163/ab9ab0.
- Silvano Dragonieri; Vitaliano N Quaranta; Pierluigi Carratu; Teresa Ranieri; Lorenzo Marra; Giuseppina D’Alba; Onofrio Resta; An electronic nose may sniff out amyotrophic lateral sclerosis. Respiratory Physiology & Neurobiology 2016, 232, 22-25, 10.1016/j.resp.2016.06.005.
- Xian-Zhe Zheng; Yu-Bin Lan; Jian-Min Zhu; John Westbrook; W. C. Hoffmann; R. E. Lacey; Rapid identification of rice samples using an electronic nose. Journal of Bionic Engineering 2009, 6, 290-297, 10.1016/s1672-6529(08)60122-5.
- Changying Li; Gerard W. Krewer; Pingsheng Ji; Harald Scherm; Stanley J. Kays; Gas sensor array for blueberry fruit disease detection and classification. Postharvest Biology and Technology 2010, 55, 144-149, 10.1016/j.postharvbio.2009.11.004.
- Federico Autelitano; Felice Giuliani; Analytical assessment of asphalt odor patterns in hot mix asphalt production. Journal of Cleaner Production 2018, 172, 1212-1223, 10.1016/j.jclepro.2017.10.248.
- Federico Autelitano; Felice Giuliani; Influence of chemical additives and wax modifiers on odor emissions of road asphalt. Construction and Building Materials 2018, 183, 485-492, 10.1016/j.conbuildmat.2018.06.168.
- Brian D. Hosfield; Anthony R. Pecoraro; Nielson T. Baxter; Troy B. Hawkins; Troy A. Markel; The Assessment of Fecal Volatile Organic Compounds in Healthy Infants: Electronic Nose Device Predicts Patient Demographics and Microbial Enterotype. Journal of Surgical Research 2020, 254, 340-347, 10.1016/j.jss.2020.05.010.
- Manuela Baietto; Alphus D. Wilson; Daniele Bassi; Francesco Ferrini; Evaluation of Three Electronic Noses for Detecting Incipient Wood Decay. Sensors 2010, 10, 1062-1092, 10.3390/s100201062.
- Alphus D. Wilson; Charisse S. Oberle; Daniel F. Oberle; Detection of Off-Flavor in Catfish Using a Conducting Polymer Electronic-Nose Technology. Sensors 2013, 13, 15968-15984, 10.3390/s131215968.
- Henike Guilherme Jordan Voss; José Jair Alves Mendes Júnior; Murilo Eduardo Farinelli; Jr. Sergio Luiz Stevan; A Prototype to Detect the Alcohol Content of Beers Based on an Electronic Nose.. Sensors 2019, 19, 2646, 10.3390/s19112646.




