
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Moon Sajid | + 2508 word(s) | 2508 | 2021-05-26 05:23:12 | | | |
| 2 | Bruce Ren | -21 word(s) | 2487 | 2021-06-04 03:20:52 | | |
Video Upload Options
Flavonoids are a structurally diverse class of natural products that have been found to have a range of beneficial activities in humans. However, the clinical utilisation of these molecules has been limited due to their low solubility, chemical stability, bioavailability and extensive intestinal metabolism in vivo. Recently, the view has been formed that site-specific modification of flavonoids by methylation and/or glycosylation, processes that occur in plants endogenously, can be used to improve and adapt their biophysical and pharmacokinetic properties. The traditional source of flavonoids and their modified forms is from plants and is limited due to the low amounts present in biomass, intrinsic to the nature of secondary metabolite biosynthesis. Access to greater amounts of flavonoids, and understanding of the impact of modifications, requires a rethink in terms of production, more specifically towards the adoption of plant biosynthetic pathways into ex planta synthesis approaches. Advances in synthetic biology and metabolic engineering, aided by protein engineering and machine learning methods, offer attractive and exciting avenues for ex planta flavonoid synthesis.
1. Introduction
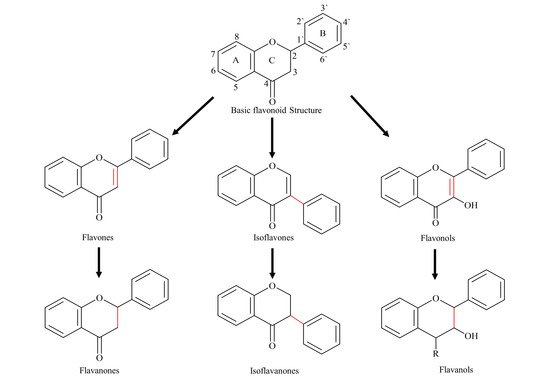
2. Pharmacokinetic Challenges of Flavonoids
| Properties | Flavonoid Characteristics | |
|---|---|---|
| Solubility | Low intestinal absorption making it difficult to attain pharmacologically effective concentration in-vivo | [12] |
| Chemical stability | Difficulties in extraction and long-term storage | [13] |
| Metabolic stability Hepatic, intestinal Intestinal Microflora |
Different substitutions on basic skeleton results in lower activity, and inertness which finally leads to excretion Intestinal microflora also results in flavonoids degradation (by hydrolysis, reduction and ring fission) |
[10][14] |
| In-planta production constraints | ||
| Yield | Very low yield of plant secondary metabolites relative to biomass Agricultural and resource constraints to produce sufficient plant biomass |
[15][16] |
| Purity | Heterogeneous mixtures difficult to assign a particular function to a specific molecule Isolation and identification of a particular compound is difficult |
[17][18] |
| Biosynthesis | Regulatory and bioengineering challenges in genetic engineering to increase yield in-planta, Seasonal variations in yield and composition |
[19] |
| Isolation and extraction | Loss in activity due to degradation and alteration in chemical structure Production of too much waste during extraction process |
[20] |
3. Flavonoids Derivative with Improved PK Characteristics
4. Methylated Flavonoids
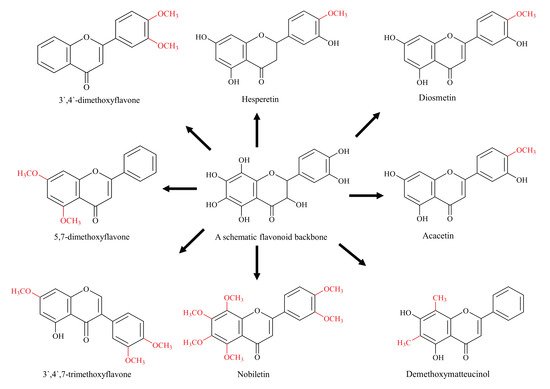
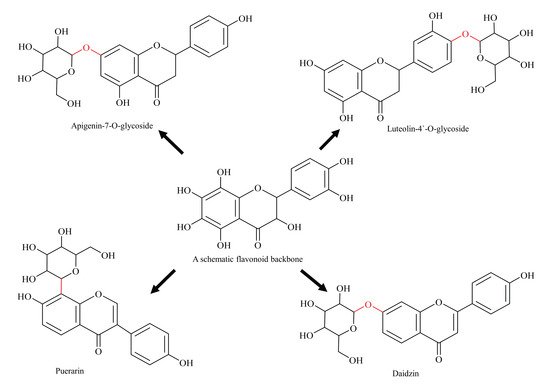
5. Glycosylated Flavonoids
References
- Ross, J.A.; Kasum, C.M. Dietary Flavonoids: Bioavailability, Metabolic Effects, and Safety. Annu. Rev. Nutr. 2002, 22, 19–34.
- Gates, M.A.; Vitonis, A.F.; Tworoger, S.S.; Rosner, B.; Titus-Ernstoff, L.; Hankinson, S.E.; Cramer, D.W. Flavonoid Intake and Ovarian Cancer Risk in a Population-based Case-control Study. Int. J. Cancer 2009, 124, 1918–1925.
- Gates, M.A.; Tworoger, S.S.; Hecht, J.L.; De Vivo, I.; Rosner, B.; Hankinson, S.E. A Prospective Study of Dietary Flavonoid Intake and Incidence of Epithelial Ovarian Cancer. Int. J. Cancer 2007, 121, 2225–2232.
- Lei, L.; Yang, Y.; He, H.; Chen, E.; Du, L.; Dong, J.; Yang, J. Flavan-3-Ols Consumption and Cancer Risk: A Meta-Analysis of Epidemiologic Studies. Oncotarget 2016, 7, 73573.
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137.
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World, J. 2013, 2013.
- Jiang, D.; Rasul, A.; Batool, R.; Sarfraz, I.; Hussain, G.; Mateen Tahir, M.; Qin, T.; Selamoglu, Z.; Ali, M.; Li, J. Potential Anticancer Properties and Mechanisms of Action of Formononetin. BioMed Res. Int. 2019, 2019.
- Sudhakaran, M.; Sardesai, S.; Doseff, A.I. Flavonoids: New Frontier for Immuno-Regulation and Breast Cancer Control. Antioxidants 2019, 8, 103.
- Ye, Q.; Liu, K.; Shen, Q.; Li, Q.; Hao, J.; Han, F.; Jiang, R.-W. Reversal of Multidrug Resistance in Cancer by Multi-Functional Flavonoids. Front. Oncol. 2019, 9, 487.
- Amawi, H.; Ashby, C.R.; Tiwari, A.K. Cancer Chemoprevention through Dietary Flavonoids: What’s Limiting? Chin. J. Cancer 2017, 36, 1–13.
- At 16.5 % of CAGR Soy Isoflavones Market Size Will Rise and Expected to Cross 3510.4 Million USD in 2025. Available online: (accessed on 31 March 2021).
- Chen, C.-Y.O.; Milbury, P.E.; Blumberg, J.B. Polyphenols in Almond Skins after Blanching Modulate Plasma Biomarkers of Oxidative Stress in Healthy Humans. Antioxidants 2019, 8, 95.
- Srivastava, J.K.; Gupta, S. Extraction, Characterization, Stability and Biological Activity of Flavonoids Isolated from Chamomile Flowers. Mol. Cell. Pharmacol. 2009, 1, 138.
- Gee, J.M.; DuPont, M.S.; Day, A.J.; Plumb, G.W.; Williamson, G.; Johnson, I.T. Intestinal Transport of Quercetin Glycosides in Rats Involves Both Deglycosylation and Interaction with the Hexose Transport Pathway. J. Nutr. 2000, 130, 2765–2771.
- Zhou, J.; Du, G.; Chen, J. Novel Fermentation Processes for Manufacturing Plant Natural Products. Curr. Opin. Biotechnol. 2014, 25, 17–23.
- Hossain, M.A.; Mizanur Rahman, S.M. Isolation and Characterisation of Flavonoids from the Leaves of Medicinal Plant Orthosiphon Stamineus. Arab. J. Chem. 2015, 8, 218–221.
- Egert, S.; Rimbach, G. Which Sources of Flavonoids: Complex Diets or Dietary Supplements? Adv. Nutr. 2011, 2, 8–14.
- Zhu, Y.; Liu, Y.; Zhan, Y.; Liu, L.; Xu, Y.; Xu, T.; Liu, T. Preparative Isolation and Purification of Five Flavonoid Glycosides and One Benzophenone Galloyl Glycoside from Psidium Guajava by High-Speed Counter-Current Chromatography (HSCCC). Molecules 2013, 18, 15648–15661.
- Falcone Ferreyra, M.L.; Rius, S.; Casati, P. Flavonoids: Biosynthesis, Biological Functions, and Biotechnological Applications. Front. Plant. Sci. 2012, 3.
- Stobiecki, M.; Kachlicki, P. Isolation and Identification of Flavonoids. In The Science of Flavonoids; Springer: New York, NY, USA, 2006; pp. 47–69. ISBN 978-0-387-28821-5.
- Yu, C.-P.; Shia, C.-S.; Tsai, S.-Y.; Hou, Y.-C. Pharmacokinetics and Relative Bioavailability of Flavonoids between Two Dosage Forms of Gegen-Qinlian-Tang in Rats. Evid. Based Complement. Alternat. Med. 2012, 2012.
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of Quercetin: Problems and Promises. Curr. Med. Chem. 2013, 20, 2572–2582.
- Mauludin, R.; Müller, R.H.; Keck, C.M. Kinetic Solubility and Dissolution Velocity of Rutin Nanocrystals. Eur. J. Pharm. Sci. 2009, 36, 502–510.
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584.
- Ueno, I.; Nakano, N.; Hirono, I. Metabolic Fate of [14C] Quercetin in the ACI Rat. Jpn. J. Exp. Med. 1983, 53, 41–50.
- Boulton, D.W.; Walle, U.K.; Walle, T. Fate of the Flavonoid Quercetin in Human Cell Lines: Chemical Instability and Metabolism. J. Pharm. Pharmacol. 1999, 51, 353–359.
- Olthof, M.R.; Hollman, P.C.; Buijsman, M.N.; Van Amelsvoort, J.M.; Katan, M.B. Chlorogenic Acid, Quercetin-3-Rutinoside and Black Tea Phenols Are Extensively Metabolized in Humans. J. Nutr. 2003, 133, 1806–1814.
- Aura, A.-M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.-M.; Poutanen, K.; Santos-Buelga, C. In Vitro Metabolism of Anthocyanins by Human Gut Microflora. Eur. J. Nutr. 2005, 44, 133–142.
- Gonthier, M.-P.; Verny, M.-A.; Besson, C.; Rémésy, C.; Scalbert, A. Chlorogenic Acid Bioavailability Largely Depends on Its Metabolism by the Gut Microflora in Rats. J. Nutr. 2003, 133, 1853–1859.
- Kim, D.-H.; Jung, E.-A.; Sohng, I.-S.; Han, J.-A.; Kim, T.-H.; Han, M.J. Intestinal Bacterial Metabolism of Flavonoids and Its Relation to Some Biological Activities. Arch. Pharm. Res. 1998, 21, 17–23.
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A.; Wahab, P.E.M.; Halim, M.R.A. Effect of Different Light Intensities on Total Phenolics and Flavonoids Synthesis and Anti-Oxidant Activities in Young Ginger Varieties (Zingiber Officinale Roscoe). Int. J. Mol. Sci. 2010, 11, 3885–3897.
- Ruenroengklin, N.; Zhong, J.; Duan, X.; Yang, B.; Li, J.; Jiang, Y. Effects of Various Temperatures and PH Values on the Extraction Yield of Phenolics from Litchi Fruit Pericarp Tissue and the Antioxidant Activity of the Extracted Anthocyanins. Int. J. Mol. Sci. 2008, 9, 1333–1341.
- Preedy, V.R. Isoflavones: Chemistry, Analysis, Function and Effects; Royal Society of Chemistry: London, UK, 2012; ISBN 1-84973-419-4.
- Gawande, S.; Kale, A.; Kotwal, S. Effect of Nutrient Mixture and Black Grapes on the Pharmacokinetics of Orally Administered (-) Epigallocatechin-3-gallate from Green Tea Extract: A Human Study. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2008, 22, 802–808.
- Nunes, T.; Almeida, L.; Rocha, J.-F.; Falcão, A.; Fernandes-Lopes, C.; Loureiro, A.I.; Wright, L.; Vaz-da-Silva, M.; Soares-da-Silva, P. Pharmacokinetics of Trans-resveratrol Following Repeated Administration in Healthy Elderly and Young Subjects. J. Clin. Pharmacol. 2009, 49, 1477–1482.
- Kim, Y.S.; Ryu, Y.B.; Curtis-Long, M.J.; Yuk, H.J.; Cho, J.K.; Kim, J.Y.; Kim, K.D.; Lee, W.S.; Park, K.H. Flavanones and Rotenoids from the Roots of Amorpha Fruticosa, L. That Inhibit Bacterial Neuraminidase. Food Chem. Toxicol. 2011, 49, 1849–1856.
- Carvalho, O.V.; Botelho, C.V.; Ferreira, C.G.T.; Ferreira, H.C.C.; Santos, M.R.; Diaz, M.A.N.; Oliveira, T.T.; Soares-Martins, J.A.P.; Almeida, M.R.; Júnior, A.S. In Vitro Inhibition of Canine Distemper Virus by Flavonoids and Phenolic Acids: Implications of Structural Differences for Antiviral Design. Res. Vet. Sci. 2013, 95, 717–724.
- Wen, L.; Jiang, Y.; Yang, J.; Zhao, Y.; Tian, M.; Yang, B. Structure, Bioactivity, and Synthesis of Methylated Flavonoids. Ann. N. Y. Acad. Sci. 2017, 1398, 120–129.
- Zou, X.-W.; Liu, Y.-C.; Hsu, N.-S.; Huang, C.-J.; Lyu, S.-Y.; Chan, H.-C.; Chang, C.-Y.; Yeh, H.-W.; Lin, K.-H.; Wu, C.-J.; et al. Structure and Mechanism of a Nonhaem-Iron SAM-Dependent C-Methyltransferase and Its Engineering to a Hydratase and an O-Methyltransferase. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 1549–1560.
- SAM (Dependent) I AM: The S-Adenosylmethionine-Dependent Methyltransferase Fold. Curr. Opin. Struct. Biol. 2002, 12, 783–793.
- Koirala, N.; Thuan, N.H.; Ghimire, G.P.; Thang, D.V.; Sohng, J.K. Methylation of Flavonoids: Chemical Structures, Bioactivities, Progress and Perspectives for Biotechnological Production. Enzyme Microb. Technol. 2016, 86, 103–116.
- Wen, X.; Walle, T. Methylated Flavonoids Have Greatly Improved Intestinal Absorption and Metabolic Stability. Drug Metab. Dispos. Biol. Fate Chem. 2006, 34, 1786–1792.
- Surichan, S.; Androutsopoulos, V.P.; Sifakis, S.; Koutala, E.; Tsatsakis, A.; Arroo, R.R.J.; Boarder, M.R. Bioactivation of the Citrus Flavonoid Nobiletin by CYP1 Enzymes in MCF7 Breast Adenocarcinoma Cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012, 50, 3320–3328.
- Wei, G.-J.; Hwang, L.S.; Tsai, C.-L. Absolute Bioavailability, Pharmacokinetics and Excretion of 5, 7, 3′, 4′-Tetramethoxyflavone in Rats. J. Funct. Foods 2014, 7, 136–141.
- Walle, T.; Wen, X.; Walle, U.K. Improving Metabolic Stability of Cancer Chemoprotective Polyphenols. Expert Opin. Drug Metab. Toxicol. 2007, 3, 379–388.
- Wen, X.; Walle, T. Methylation Protects Dietary Flavonoids from Rapid Hepatic Metabolism. Xenobiotica 2006, 36, 387–397.
- Katayama, K.; Masuyama, K.; Yoshioka, S.; Hasegawa, H.; Mitsuhashi, J.; Sugimoto, Y. Flavonoids Inhibit Breast Cancer Resistance Protein-Mediated Drug Resistance: Transporter Specificity and Structure–Activity Relationship. Cancer Chemother. Pharmacol. 2007, 60, 789–797.
- Walle, T.; Ta, N.; Kawamori, T.; Wen, X.; Tsuji, P.A.; Walle, U.K. Cancer Chemopreventive Properties of Orally Bioavailable Flavonoids—Methylated versus Unmethylated Flavones. Biochem. Pharmacol. 2007, 73, 1288–1296.
- Zhang, J.; Wu, Y.; Zhao, X.; Luo, F.; Li, X.; Zhu, H.; Sun, C.; Chen, K. Chemopreventive Effect of Flavonoids from Ougan (Citrus Reticulata Cv. Suavissima) Fruit against Cancer Cell Proliferation and Migration. J. Funct. Foods 2014, 10, 511–519.
- Fan, X.; Bai, J.; Zhao, S.; Hu, M.; Sun, Y.; Wang, B.; Ji, M.; Jin, J.; Wang, X.; Hu, J. Evaluation of Inhibitory Effects of Flavonoids on Breast Cancer Resistance Protein (BCRP): From Library Screening to Biological Evaluation to Structure-Activity Relationship. Toxicol. In Vitro 2019, 61, 104642.
- Varki, A. Biological Roles of Glycans. Glycobiology 2017, 27, 3–49.
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H. Essentials of Glycobiology [Internet]; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2015.
- Desmet, T.; Soetaert, W.; Bojarová, P.; Křen, V.; Dijkhuizen, L.; Eastwick-Field, V.; Schiller, A. Enzymatic Glycosylation of Small Molecules: Challenging Substrates Require Tailored Catalysts. Chem. Eur. J. 2012, 18, 10786.
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the Biotechnological Glycosylation of Valuable Flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156.
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New Insights on Bioactivities and Biosynthesis of Flavonoid Glycosides. Trends Food Sci. Technol. 2018, 79, 116–124.
- Treml, J.; Šmejkal, K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738.
- Imai, Y.; Tsukahara, S.; Asada, S.; Sugimoto, Y. Phytoestrogens/Flavonoids Reverse Breast Cancer Resistance Protein/ABCG2-Mediated Multidrug Resistance. Cancer Res. 2004, 64, 4346–4352.
- Rüfer, C.E.; Bub, A.; Möseneder, J.; Winterhalter, P.; Stürtz, M.; Kulling, S.E. Pharmacokinetics of the Soybean Isoflavone Daidzein in Its Aglycone and Glucoside Form: A Randomized, Double-Blind, Crossover Study. Am. J. Clin. Nutr. 2008, 87, 1314–1323.
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The Biological Activities, Chemical Stability, Metabolism and Delivery Systems of Quercetin: A Review. Trends Food Sci. Technol. 2016, 56, 21–38.
- Xue, H.-F.; Ying, Z.-M.; Zhang, W.-J.; Meng, Y.-H.; Ying, X.-X.; Kang, T.-G. Hepatic, Gastric, and Intestinal First-Pass Effects of Vitexin in Rats. Pharm. Biol. 2014, 52, 967–971.
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367.




