1. Introduction
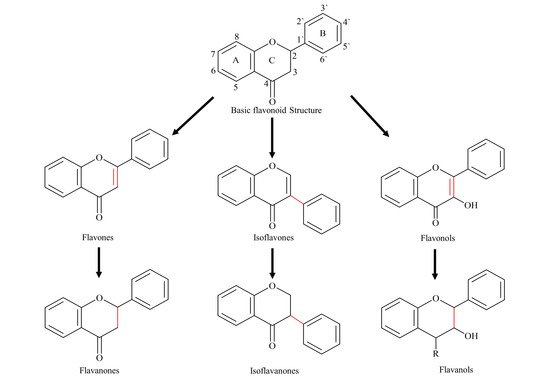
Flavonoids are one the most abundant and broadly distributed families of biologically active plant natural products (PNP). Chemically, flavonoids have a C6-C3-C6 skeleton, with two phenyl aromatic rings (A and B) along with a heterocyclic ring (C-ring). Based on the substitution of the basic skeleton and B-ring attachment, flavonoids have been split into several subclasses, for example, flavanones, flavones, flavonols, flavan-3-ols, isoflavones, and isoflavanones ()
[1]. Over 15,000 flavonoids have been identified to date, from many plant families, mainly from legumes.
Figure 1. Basic flavonoid backbone and structure of most common sub-classes.
The role of isoflavones as chemopreventive compounds has been well-established. A number of epidemiological studies, along with retrospective meta-analysis and prospective observational studies, have established that flavonoids possess anticancer activities
[2][3][4][5][2,3,4,5]. As well as these, flavonoids possess a range of pharmacological effects as well as antimicrobial, antioxidant and cardioprotective properties
[6]. Structural similarities with estrogen hormone and resulting interactions with cellular signaling cascades make flavonoids an interesting class to be pursued for drug discovery
[7].
Several flavonoids have been analysed for their anticancer activities, both in vitro and in vivo, including daidzein, quercetin, silymarin, luteolin, kaempferol, and apigenin
[8]. These compounds are active against prostate, colorectal, breast, thyroid, lung and ovarian cancer, including others
[9]. Flavonoids mediate their function in multiple ways: (i) by preventing the development of new cancer cells, (ii) by restraining the carcinogens from reaching their activation sites, (iii) by the reduction in toxicity of some compounds by preventing their metabolism
[10]. The underlying mechanisms by which flavonoids mediate their function has been well-established for multiple pathways.
Interest in flavonoids production is growing, which is reflected by their 16.5% projected CAGR, increasing the current market from USD 1.9 billion in 2019 to USD 3.5 billion by 2025
[11]. Although the role of flavonoids in the prevention of cancer has been well established, their low availability, issues in isolation and purification of a specific targeted compound, and limited understanding regarding absorption and intestinal metabolism have held back the development of flavonoids as approved drugs for clinical use
[10]. It is well known that post-synthesis modifications, such as methylation, glycosylation, phosphorylation and alkylation, all impact pharmacokinetics (PK) and pharmacodynamics (PD). Plants already utilise this chemical space and these modified compounds may be the answer to the challenges facing the clinical usage of flavonoids. These modifications, which are otherwise difficult to access from traditional synthetic chemistry, can be accessed by applying synthetic biology methodologies and metabolic engineering to produce flavonoids in microorganisms.
2. Pharmacokinetic Challenges of Flavonoids
Many pharmacological functions are associated with flavonoids; however, a number of problems are holding back their development as approved drugs for clinical use and, to some extent, further research studies (). Some of them are their low solubility, bioavailability and, to some extent, low yield in host plants, which are discussed in the following sections; however, certain others, like issues in purification from plant sources, and problems in conducting reliable epidemiological studies, have been discussed elsewhere
[10].
Table 1. Chemical and biophysical properties, and challenges of flavonoids in planta production.
| Properties |
Flavonoid Characteristics |
|
| Solubility |
Low intestinal absorption making it difficult to attain pharmacologically effective concentration in-vivo |
[12] |
| Chemical stability |
Difficulties in extraction and long-term storage |
[13] |
Metabolic stability
Hepatic, intestinal
Intestinal Microflora |
Different substitutions on basic skeleton results in lower activity, and inertness which finally leads to excretion
Intestinal microflora also results in flavonoids degradation (by hydrolysis, reduction and ring fission) |
[10][14] | [10,14] |
| In-planta production constraints |
|
| Yield |
Very low yield of plant secondary metabolites relative to biomass
Agricultural and resource constraints to produce sufficient plant biomass |
[15][16] | [15,16] |
| Purity |
Heterogeneous mixtures difficult to assign a particular function to a specific molecule
Isolation and identification of a particular compound is difficult |
[17][18] | [17,18] |
| Biosynthesis |
Regulatory and bioengineering challenges in genetic engineering to increase yield | in-planta | ,
Seasonal variations in yield and composition |
[19] |
| Isolation and extraction |
Loss in activity due to degradation and alteration in chemical structure
Production of too much waste during extraction process |
[20] |
The absorption and metabolism of flavonoids have been extensively studied in the last two decades. Generally, the PK profile (i.e., absorption, distribution, metabolism, excretion and toxicity) of flavonoids is not optimal and varies considerably across different classes
[21]. Flavonoids generally possess low bioavailability when orally administered which significantly decreases their chance of attaining effective concentration in vivo
[22]. The reasons for this are their low solubility, poor oral absorption, and extensive hepatic metabolism by phase-I and II enzymes
[23].
Intestinal metabolism also affects the absorption of flavonoids via chemical reactions occurring in epithelial cells of the small intestine and/or mediated by the intestinal microbiota
[14]. Flavonoids are substrates for glucuronidation, O-methylation and sulfation in small-intestine epithelial cells and these chemical modifications decrease the bioactivity of flavonoids, meaning that these metabolites are excreted
[24]. For example, following oral administration in rats, only 20% of quercetin was absorbed in the intestine; the rest was decomposed to CO
2 as well as excreted in the feces. On the other hand, the absorbed quercetin was also excreted out of the body within 48 h
[25]. Quercetin has a low stability profile, as it is degraded within 6 h of incubation under normal physiological conditions (Hanks’ Balanced Salt solution, pH 7.4)
[26]. Furthermore, when the unabsorbed flavonoids reach the colon, they undergo degradation into different metabolites by intestinal microflora, mostly via hydrolysis, reduction or ring fission
[27][28][29][30][27,28,29,30].
Surprisingly, the PK profile of flavonoids is also compromised in planta due to environmental factors like light, temperature, oxygen exposure, pH and ultraviolet radiation. Light exposure, specifically UV light, can alter the biosynthesis of flavonoids in host plants. For example, the antioxidant activity of total flavonoids isolated from plant
Halia bara was optimum at a wavelength of 310 umolm
−2s
−1 and any variation in this wavelength results in reduced biosynthesis and bioactivity of flavonoids
[31]. Temperature also plays an important role in the extraction and shelf-life of flavonoids. For instance, the optimal temperature for the extraction and purification of flavonoids from pericarp tissue of litchi fruit is 45–60 °C, and other temperatures result in significant yield loss and degradation
[32]. As environmental factors are difficult to control and manage, it is impossible to predict the yield and biological activity of flavonoids present in plant extracts.
Flavonoids are present in very minute quantities (micro/milligram per kg of plant biomass) in plant hosts. Therefore, continuous extraction from plant sources, on the one hand, strains an already vulnerable agriculture sector and, on the other hand, might result in price hikes due to unstable supply and demand issues
[17]. Flavonoids are commonly available as a plant extract that is a mixture of many plant natural products, therefore making it difficult to link a particular pharmacological effect with a specific flavonoid compound
[10]. The presence of multiple secondary metabolites in crude mixture also makes it difficult to isolate and identify a compound of interest. Along with this, isolation and purification methods are costly, hazardous and multistage processes that further decrease the final yield
[18]. Taking all these factors into account, it becomes very difficult to predict the final yield, and sometimes makes it impossible to maintain a sustainable supply of required compounds on the market
[33]. Therefore, the extraction of flavonoids from plant sources is time-consuming, cost-inefficient, gives very low yield and produces much waste.
Chemical stability, PK issues and low availability are significant obstacles to the clinical development of flavonoids as effective chemopreventive therapy, because the required in vivo level is almost impossible to achieve, even with high oral doses
[34][35][34,35]. However, there is hope, as a few reports have shown that certain substitutions for flavonoid, i.e., methylation, glycosylation, can improve the PK profile of flavonoids. The roles of methylation and glycosylation are further explained in the following paragraphs and, in the next section, we will shed light on synthetic biology approaches that can help to improve/solve the issues of bioavailability and pharmacokinetics faced by flavonoids.
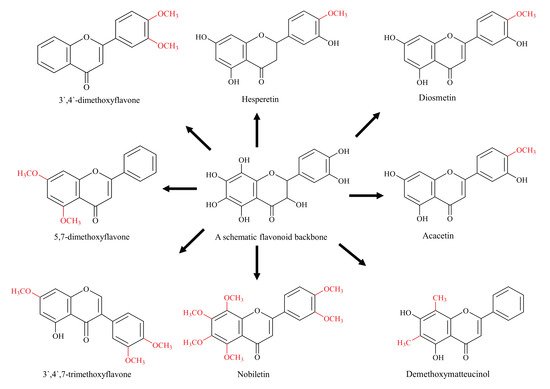
4. Methylated Flavonoids
Methylation, the addition of a methyl group to a substrate, controls several important functions of cells, from gene regulation through epigenetics to maintaining cellular energy status
[38]. Depending upon the site, methylated flavonoids are divided into two types: O-methylated flavonoids, ones that obtain a methyl group through hydroxyl group and C-methylated flavonoids, in which the methyl group is directly bound to C atoms of the basic skeleton (). Both the O-methylation and C-methylation reactions are catalysed by their respective O-methyltransferases (OMT) and C-methyltransferases (CMT). The methyl group is usually donated by an electrophilic
S-adenosyl-L-methionine (SAM) through a biomolecular nuclear substitution (SN2) reaction
[38][39][38,39]. Catechol-OMT, a caffeic acid methyltransferase, is the first SAM-dependent methyltransferase to be crystallized and represented a critical milestone in drug research
[40].
Figure 2. Diagrammatic representation of methylated flavonoids (schematic flavonoid is a hypothetical compound used to show all hydroxyl positions accessible for methylation).
The role of methylation in improving the metabolic stability of flavonoids has been documented
[41]. O-methylated flavonoids have better bioavailability because of its better absorption and increased permeability across membranes
[42][43][44][42,43,44]. In one study, over 8–10-fold better intestinal absorption was documented for methylated flavonoids compared with their non-methylated parent molecules
[42]. Methylation is considered to be the main reason behind for the absorption and improved stability. The improved metabolic stability is mainly based on the hypothesis that the blocking of flavonoid-free hydroxyl groups can help in reducing the conjugation reaction by glucuronidation and sulfation, the primary factors responsible for the poor bioavailability and stability of flavonoids
[45]. The case of galangin and its methylated derivatives is another interesting example in support of this hypothesis, where it was reported that nearly 90% of galangin was metabolised by glucuronidation and sulfation within 1 h of incubation
[46]. On the other hand, the methylated derivatives, 3′,4′-dimethoxyflavone, showed great resistance to metabolism and 5,7-dimethoxyflavone remained relatively unaffected, showing the improved metabolic stability of methylated flavonoids
[46].
Methylated derivatives of flavonoids usually show higher bioactivity, and the site as well as extent of methylation play an important role. B-ring methylation appears to have greater anti-cancer potential, where a study of over 30 different flavonoids showed that methylation of 4-C of the B-ring is linked with higher potency
[47]. For example, hesperetin is a stronger inhibitor as compared with the eriodictyol, diosmetin is more active than luteolin, acacetin is a strong inhibitor than apigenin and kaempferide is more potent than kaempferol when tested against breast cancer resistance protein (BCRP) in the
BCRP-transduced human-leukemia K562 cells
[47]. This phenomenon is also observed with methylated forms of chrysin and apigenin, which showed 10 and 8 times more potency against oral squamous carcinoma than their non-methylated parent compounds
[48]. Similarly, nobiletin and tangeretin (polymethoxylated isoflavones) have shown higher proliferative inhibition among many Ougan flavonoids
[49]. The authors have concluded that 3′-O-methylation is also linked with the enhanced anti-proliferative function of nobiletin. Similarly, a comparatively more methylated flavonoid 3′,4′,7-trimethoxyflavone has a stronger inhibitory effect on BCRP as compared with less methylated acacetin, which is one of the strongest BCRP inhibitor flavonoid
[47][50][47,50]. These are few examples highlighting methylated flavonoids as more stable, potent and bioavailable chemotherapeutic agents compared to their non-methylated analogs.
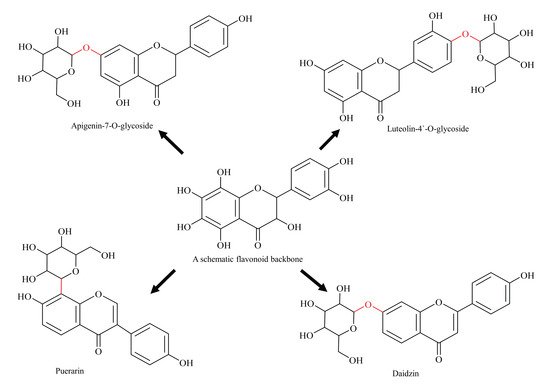
5. Glycosylated Flavonoids
Glycosylation affects physicochemical properties, immunogenicity, and PK/PD characteristics of chemical compounds
[51][52][51,52]. The glycosylation of flavonoids to form O-, or C-linked glycosides is viewed as a general route to address issues of poor solubility, stability, and toxicity and, further, is an area of intense research
[53][54][53,54]. Glycosylated flavonoids are categorised as O-glycosides or C-glycosides based on the type of glycosidic bond with the flavonoid basic skeleton (). In case of O-glycosides, the sugar moiety is attached to the basic skeleton via hydroxyl bond (commonly at 3-C and 7-C hydroxyl positions), and, in the case of C-glycoside, the sugar molecule is linked to the flavonoid basic skeleton by their respective carbon atoms (commonly at C-6 and C-8 positions)
[55]. Generally, O-glycosylation is common in flavones and flavanols sub-classes and C-glycosylation is common in flavones sub-class
[54].
Figure 3. Diagrammatic representation of glycosylated flavonoids (schematic flavonoid is a hypothetical compound used to show all hydroxyl positions accessible for glycosylation).
Flavonoid glycosides are generally soluble in water and alcohol; however, a few flavonoids, such as rutin and hesperidin, are sparingly soluble. Conversely, aglycans (non-glycosylated parent flavonoid) usually dissolve in non-polar solvents
[56]. As well as this, glycosylation increases the chemical stability of flavonoids in vitro. For instance, Srivastava and Gupta have reported that Chamomile glycosides (dominantly apigenin-7-
O-glucoside) were highly stable in their solutions under a range of storage conditions (temperature, pH and solvent)
[13]. Improvement in stability is a desirable clinical characteristic, and thus glycosylated flavonoids are viewed with great promise.
Anti-BCRP activity has also been observed for a few glycosylated flavonoids, for example, apigenin-7-glucoside and luteolin-4′-
O-glucoside and the possible reason for this might be their better water-solubility and higher absorption
[57]. In another study, it was documented that glycosylated flavonoid (daidzin), when ingested in pure form, has higher systemic bioavailability and plasma concentration compared with non-glycosylated parent flavonoid (daidzein) in healthy men
[58]. Thus, glycosylation improves the bioavailability of flavonoids, and helps them to retain their native skeleton, which results in a higher inhibitory effect; therefore, such findings are viewed with great importance
[57][58][57,58]. On the other hand, aglycans, being insoluble in water, are difficult to administer and, therefore, glycosylated flavonoids are better options.
On the other hand, in some cases, glycosylated flavonoids tend to show more variable bioavailability than their aglycans
[59][60][59,60]. This was explained by the literature on the flavonoid quercetin, where different glycosides show varied absorption rates and absorption sites, with quercetin-4′-
O-glucoside having metabolites which are five times more available compared to the metabolites of quercetin-3-
O-rutinoside
[61]. Similarly, not all glycosylated flavonoids have shown higher anti-BCRP activity compared with their aglycans, which indicates that glycosylation has variable effects
[57]. Therefore, the basic skeleton of flavonoids, as well as the specific sites of attachment and type of sugar unit, play a role in determining the possible pharmacological outcome and, to keep the effects of glycosylation simple to understand, we have mainly considered glucosylated flavonoids as examples.