
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Felipe Sanchez Bragagnolo | + 2776 word(s) | 2776 | 2021-06-18 06:49:25 | | | |
| 2 | Conner Chen | Meta information modification | 2776 | 2021-06-28 15:43:55 | | |
Video Upload Options
Soy has been recognized as a medicinal plant since it contains several bioactive compounds in its various parts. For example, bioactive peptides found in soybeans have been linked to human health benefits with potential anti-hypertensive, anti-cancer, and anti-inflammatory properties. Another type of bioactive compound identified in soybeans, the anthocyanins, showed anti-obesity and anti- inflammatory properties. Isoflavonoids, the best-known class of compounds found in all parts of soy, have been studied due to their potential protective effects associated with chronic diseases, cancer, osteoporosis, and menopausal symptoms. Different factors modulate a plant’s metabolism, and metabolomics can measure these variations qualitatively and quantitatively, analyzing the production and turnover of primary and secondary (specialized) metabolites. In soy, metabolomics studies have identified four main causes of changes in metabolism: genetic modifications, organism interactions, growth stages, and abiotic factors.
1. Metabolomics Applied to Agri-Foods and Their By-Products
Plants have been used to produce food, feed, energy, biomaterials, and also as a source of bioactive compounds. Metabolomics has emerged as one of the principal contributors to enhancing the identification of these compounds, generating innovative discoveries and supporting the development of novel products [1]. Progress in efficient extraction techniques, such as ultrasound, microwave, and pulsed-electric-field-assisted extractions, as well as supercritical fluid and pressurized liquid extractions, among others, generate extracts with a higher yield and bioactivity [2][3][4][5][6]. Once these extracts are generated, they can be analyzed with one or more powerful chromatography and/or electrophoresis techniques coupled to high-resolution mass spectrometry (MS) or nuclear magnetic resonance (NMR), producing accurate chemical information on a vast number of compounds [7][8][9][10][11][12]. For the identification of metabolites, databases have been increasingly updated, crosslinking information from different libraries. Sorokina and Steinbeck [13] list almost one hundred databases useful for natural product research. In addition, Global Natural Product Social Molecular Networking (GNPS) and Small Molecule Accurate Recognition Technology (SMART 2.0) are examples of bio-cheminformatics tools for the analysis of MS and NMR data, respectively [14][15][16]. All these modern techniques and tools support the advancement of metabolomics’ frontiers.
In 2019, 8.3 billion metric tons of cereals, oil crops, roots and tubers, sugar crops, and vegetables were produced [17]. However, it is estimated that one-third of food production is lost and wasted, and this problem is Target 12.3 of the 17 Sustainable Development Goals (SDGs) set by the United Nations (UN) [18][19][20]. In this context, foodomics has shown the potential not only of foods, but also of their related by-products, as sources of compounds with human health benefits (Figure 1) [21][22]. For example, Katsinas et al. [23] used supercritical carbon dioxide and pressurized liquid extractions to valorize olive pomace, which is a by-product of the olive oil industry. As a result, they identified several phenolic compounds and generated bioactive extracts. Assirati et al. [24] applied a metabolomics approach in the chemical investigation of the three major solid sugarcane (Saccharum officinarum) by-products, leading to the identification of up to 111 metabolites in a single matrix, with several of these compounds already known by their potent bioactive properties, such as 1-octacosanol, octacosanal, orientin, and apigenin-6-C-glucosylrhamnoside. Terpenes of orange (Citrus sinensis) juice by-products showed antioxidant and neuroprotective potential in in vitro assays, as revealed by Sánchez-Martínez et al. [25]. As for the permeability of the blood–brain barrier, some terpenes of orange extract demonstrated a high capacity to cross this obstacle, which is a critical point for treating Alzheimer’s disease [25][26].
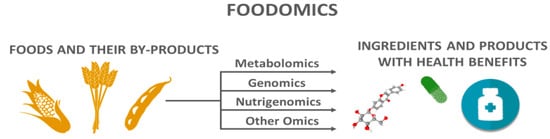
Figure 1. Foodomics proposes a holistic approach to develop ingredients and products with health benefits from foods and their by-products.
2. Glycine max: More Than Beans
Soy, also known as soybean (Glycine max (L.) Merr.), is originally from China and Eastern Asia [27]. It is the major oilseed crop worldwide, with a world production of 362, 254, and 61 million metric tons of soy grains, meal, and oil, respectively, in 2020/21. For the same period, the global area harvested was 1.28 million km2, 2.5 times the area of Spain [28][29]. Figure 2 shows soybean production from 2000/01 to 2020/21, demonstrating consistent growth, with few moments of decrease [29][30]. However, this production involves just one part of G. max: the beans. Krisnawati and Adie [31] analyzed 29 soybean genotypes and found an average value of 1.65 for the straw:grain ratio in soy. Therefore, it is estimated that about 597 million metric tons of soy branches, leaves, pods, and roots will be left on the ground post-harvesting in 2020/21 [29][31]. Figure 3 shows the soil of a no-tillage soybean production, a system which leaves all underused soy parts on the ground. Keeping these materials on the soil contributes to mineral, organic matter, and humidity factors [32]. In contrast, problems related to higher weed and disease infestations, as well as greenhouse gas emissions caused by the decomposition of organic matter, require alternative management of the agricultural straw [33][34][35][36][37][38]. By applying a biorefinery approach, such by-products could be transformed into raw material for the extraction of several bioactive compounds.
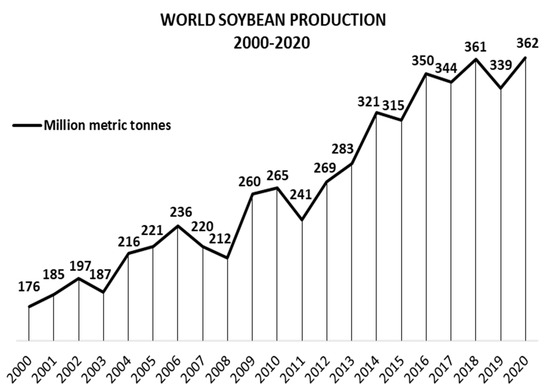
Figure 2. World soybean production 2000–2020, in million metric tons.
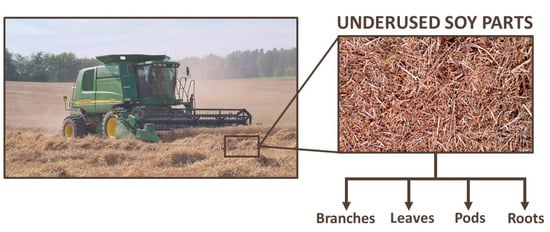
Figure 3. Underused soy parts left on the soil just after the soybean harvest.
Inspired by the potential of underused soy parts, this review aims to show the application of metabolomics in soy analysis, listing the potential of these by-products as a source of high-added-value compounds, as well as the factors which affect their production.
3. Metabolomics and Soy
Genetic modifications can be related to different species and cultivar/variety of soybean. Lu et al. [39] investigated the metabolic changes between two soybean species (Glycine max and Glycine soja) under salt stress. Using gas chromatography coupled to mass spectrometry (GC–MS) and liquid chromatography coupled to Fourier transform and mass spectrometry (LC–FT/MS), the authors found a higher content of hormones, reactive oxygen species, and other substances related to the salt stress condition. In another study, Glycine max and Glycine gracilis presented different profiles of secondary metabolites during the growth stage, as revealed by a 1H NMR-based metabolomics approach [40]. The advancement of molecular biology provides the development of a wide range of soybean cultivars or varieties, with new types of plants resistant against insects, abiotic stress, and other factors. The United States Patent and Trademark Office (USPTO) database reveals 4869 patents for a “soybean cultivar” or “soybean variety” search [41]. Different colored soybeans, such as brown, yellow, or black, present specific metabolite profiles [42][43][44]. Isoflavones could be the substrate for the production of proanthocyanidin in the seed coat, being a possible cause for the brown color of the cultivar Mallikong mutant [43]. Yang et al. [44] identified higher levels of anthocyanin and protein in yellow cotyledon seeds of black soybean. In contrast, higher levels of isoflavone, stearic acid, and polysaccharide are related to the green cotyledon seeds of the same species. Two Korean soy cultivars, Sojeongja and Haepum, presented different levels of soyasaponins Aa and Ab, whose production is related to specific gene variations [45]. Another important factor in genetic modification is the transgenic soybean. García-Villalba et al. [46] used capillary electrophoresis time-of-flight mass spectrometry (CE-TOF-MS) to qualitatively and quantitatively measure the metabolites of transgenic and non-transgenic soybeans. In summary, similar types and amounts of metabolites were identified. The same result was achieved by Harrigan et al. [47] and Clarke et al. [48]. However, it is reported that transgenic soybeans were less affected by generational effects and can present more secondary metabolites, such as prenylated isoflavones [49][50].
Moreover, the interaction between soy and microorganisms, nematodes, aphids, and other insects causes distinct metabolic responses, and metabolomics is a unique approach for understanding such changes, providing insights to improve soy’s response against biotic factors [51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68]. Recent works used GNPS to identify metabolite variation in soy infected by the fungus Phakopsora pachyrhizi and the nematode Aphelenchoides besseyi [66][67][68]. Both pathogens resulted in a higher production of bioactive compounds such as flavonoids, isoflavonoids, and terpenoids.
Distinct metabolic responses have also been reported for each growth stage of soybean [69][70][71]. During germination, 58 metabolites were reported in the separation of soy sprouts, such as phytosterols, isoflavones, and soyasaponins [72]. The production of secondary metabolites such as daidzein, genistein, and coumestrol also changed in the vegetative and reproductive soybean stages, as described by Song et al. [73].
The presence of soybean crops in a wide range of latitudes and longitudes is a consequence of several adaptive changes in their metabolism. Brazil, which is the major producer of soybean, presents different soil and climate types; even so, there is soy production in all its regions. This fact corroborates the high performance of soybean in several abiotic conditions. In addition, treatments with fertilizers and other agricultural inputs have been tested for the cultivation of soybeans in unfavorable conditions, causing additional modifications in soy metabolism [74][75][76][77][78][79][80][81][82]. As an example of external treatments, ethylene application on soybean leaves increased the genistin, daidzin, malonylgenistin, and malonyldaidzin production [82]. Using two ionization methods, electrospray ionization (ESI) and matrix-assisted laser desorption ionization (MALDI), coupled to Fourier transform ion cyclotron resonance-mass spectrometry (FTICR-MS), Yilmaz et al. [83] analyzed the metabolite profile of soy leaves from midsummer to autumn. They found a decreased production of chlorophyll-related metabolites and a higher level of disaccharides from summer to autumn. Another metabolomic approach analyzed soy leaves from crops with different geographical localizations and identified different amounts of metabolites such as pinitol and flavonoids [84]. An excellent review performed by Feng et al. [85] summarizes the use of metabolomics in soy under abiotic stress.
4. Bioactive Compounds in Underused Soy Parts
In addition to the four main causes of change in soy metabolism mentioned above, both qualitative and quantitative metabolic variations among soy organs are expected. To present an overview of the metabolite profile of underused soy parts, we selected metabolomics and related works which used various approaches to analyze them [38][55][66][67][68][81][82][86][87][88][89][90][91][92][93][94][95][96]. Using Jchem (JChem for Excel 21.1.0.787, ChemAxon (https://www.chemaxon.com, accessed on 8 April 2021)) [97] and ClassyFire [98], we organized and classified the metabolites identified in soy roots, leaves, branches, and pods, respectively. Figure 4 summarizes the best-known classes of bioactive compounds identified in underused soy parts. Carboxylic acids and their derivatives, such as amino acids, peptides, and analogues, are the most mentioned class of compounds. This class is mainly composed of primary metabolites; however, it also contains several bioactive compounds. Similarly, organooxygen and fatty acyl compounds include metabolites with human health benefits. Isoflavonoids, which are the most mentioned class of secondary metabolites, as well as prenol lipids and flavonoids, have been suggested to have a wide range of medicinal uses. Focusing on secondary metabolites, prenol lipids are the most identified class of compounds in soy roots, with several soyasaponins found in this part. In soy leaves, different subclasses of isoflavonoids have been found, such as isoflavonoid O-glycosides, isoflavans, isoflav-2-enes, and others. The metabolite profiles of soy branches and pods have been less studied; however, approximately 20 flavonoids and isoflavonoids have been identified in each part. Other classes of compounds, such as steroids and steroid derivatives, coumarins and derivatives, and cinnamic acids and derivatives, have been found in underused soy parts.
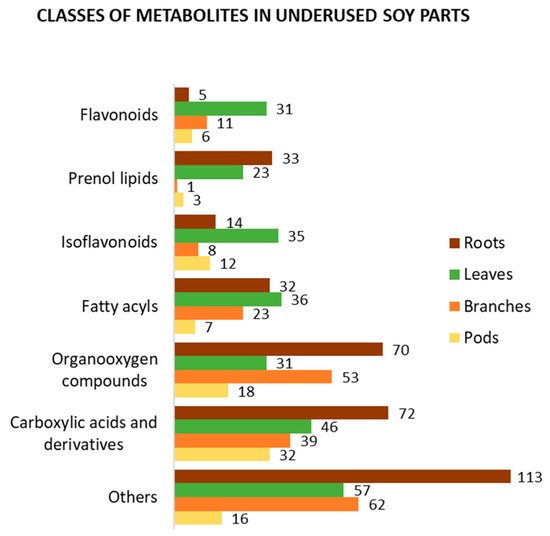
Figure 4. Classification of the metabolites identified in soy roots, leaves, branches, and pods according to ClassyFire.
Table 1 presents 38 isoflavonoids identified in one or more of the above-mentioned underused soy parts. Eight of them (daidzein, genistein, glycitein, daidzin, genistin, glycitin, malonyldaidzin, and malonylgenistin) were reported in all soy organs. Recent works showed promising biological activities of daidzein against colon cancer and hepatitis C virus [99][100]. Daidzin, which is a glyco-conjugate form of daidzein, presented therapeutic properties against multiple myeloma and epilepsy [101][102]. Bioactivity studies regarding the other aforementioned compounds also found properties against chronic vascular inflammation, human gastric cancer, breast cancer, and degenerative joint diseases [103][104][105]. Biochanin A, coumestrol, glyceollin, medicarpin, and ononin are more examples of widely known bioactive isoflavonoids which are found in different soy organs (see Table 1 for a summary) [106][107][108][109][110]. Carneiro et al. [38] quantified six isoflavones in soy branches, leaves, pods, and beans collected just after mechanical harvesting. Almost 3 kg of isoflavones were found per metric ton of soy leaves. However, less than 1 kg per metric ton was found in soy branches and pods. In soybeans, which are the main product of the soy plant, it was approximately 2 kg per metric ton.
Table 1. Isoflavonoids identified in soy branches (B), leaves (L), pods (P), and roots (R).
| Name | Formula | B | L | P | R | References |
|---|---|---|---|---|---|---|
| 2′-hydroxydaidzein | C15H10O5 | X | [68] | |||
| 7,3′,4′-trihydroxyisoflavone | C15H10O5 | X | [67] | |||
| 7-O-methylluteone | C21H20O6 | X | [66] | |||
| acetyl daidzin | C22H22O9 | X | [92] | |||
| acetyl genistin | C23H22O11 | X | X | [82][92] | ||
| acetyl glycitin | C24H24O11 | X | [92] | |||
| afrormosin 7-O-glucoside | C23H24O10 | X | [68] | |||
| biochanin A | C16H12O5 | X | [68] | |||
| biochanin A 7-O-D-glucoside | C22H22O10 | X | [68] | |||
| biochanin A 7-O-glucoside-6′′-O-malonate | C25H24O13 | X | [68] | |||
| calycosin | C16H12O5 | X | [68] | |||
| coumestrol | C15H8O5 | X | X | [67][68][89] | ||
| daidzein | C15H10O4 | X | X | X | X | [38][66][67][68][82][86][89][91][92][94][95][96] |
| daidzin | C21H20O9 | X | X | X | X | [38][66][67][68][82][86][89][91][92][95][96] |
| formononetin | C16H12O4 | X | [68][90] | |||
| formononetin 7-O-glucoside | C22H22O9 | X | X | [67][68] | ||
| formononetin 7-O-glucoside-6′′-malonate | C25H24O12 | X | [66][68][82] | |||
| formononetin 7-O-glucoside-6-O-malonate | C25H24O12 | X | X | [66][67] | ||
| genistein | C15H10O5 | X | X | X | X | [38][67][82][86][92][96] |
| genistin | C21H20O10 | X | X | X | X | [38][66][67][82][89][92][95][96] |
| glyceollidin I/II | C20H20O5 | X | [68] | |||
| glyceollin I | C20H18O5 | X | [66][68] | |||
| glyceollin II | C20H18O5 | X | [66][68] | |||
| glyceollin III | C20H18O5 | X | [66][68] | |||
| glyceollin IV | C21H22O5 | X | [68] | |||
| glyceollin VI | C20H16O4 | X | [68] | |||
| glycitein | C16H12O5 | X | X | X | X | [38][68][86][92][96] |
| glycitein 7-O-glucoside | C22H22O10 | X | [68] | |||
| glycitin | C22H22O10 | X | X | X | X | [38][67][89][92][96] |
| isotrifoliol | C16H10O6 | X | [68] | |||
| malonyldaidzin | C24H22O12 | X | X | X | X | [38][66][67][68][82][89][91][92][95][96] |
| malonylgenistin | C24H22O13 | X | X | X | X | [66][67][68][82][89][92][95][96] |
| malonylglycitin | C25H24O13 | X | X | X | [68][82][92][96] | |
| medicarpin | C16H14O4 | X | [68] | |||
| neobavaisoflavone | C20H18O4 | X | X | [66][67] | ||
| phaseollin | C20H18O4 | X | [68] | |||
| pisatin | C17H14O6 | X | [68] | |||
| sojagol | C20H16O5 | X | [66][68] |
4.1. Roots
Different compounds belonging to the prenol lipids category, which are recognized by their bioactivity, have already been identified in soy. Tsuno et al. [96] identified several soyasaponins, sapogenins, and isoflavones in soy root exudates. Soyasaponins have been linked to anti-obesity, anti-oxidative stress, and anti-inflammatory properties, as well as preventive effects on hepatic triacylglycerol accumulation [111][112][113][114]. Omar et al. [115] identified the potent inhibitory effects of soyasapogenol A, which is a triterpenoid, against p53-deficient aggressive malignancies. In addition, other compounds of different classes, such as fatty acyls, isoflavonoids, flavonoids, and others. Linoleic acid, naringenin, and formononetin-7-O-glucoside, which are examples of the aforementioned classes, have been related to cardiovascular health, neuroprotective effects, and anti-inflammatory properties [110][116][117]. The chemical structures of these bioactive compounds are presented in Figure 5.
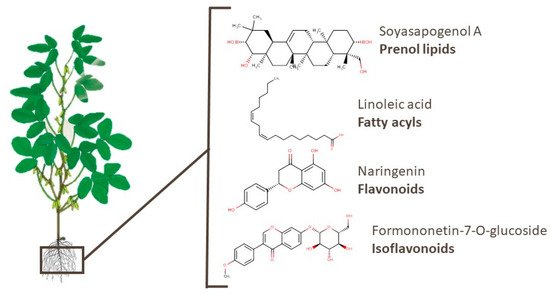
Figure 5. Chemical structures of soyasapogenol A, linoleic acid, naringenin, and formononetin-7-O-glucoside, which are examples of bioactive compounds identified in soy roots.
4.2. Leaves
Leaves and roots are the most-studied underused soy parts. 259 metabolites of 32 classes identified in soy leaves are presented [38][66][68][82][86][89][90][91][94]. Almost 90 of these compounds are flavonoids, isoflavonoids, or prenol lipids. Widely known bioactive flavonoids such as apigenin, kaempferol, rutin, and others were also identified. Apigenin has been suggested as a potential anticancer agent [118]. Glyceollin I and soyasaponin I, an isoflavonoid and a prenol lipid, presented activities against breast cancer and Parkinson’s disease, respectively [108][119]. Moreover, different soyasaponins and even trigonelline, which is an alkaloid, were found in this part of the plant. For example, the latter substance was reported to have potential for lung cancer therapy, memory function recovery, and an anti-obesity effect [120][121][122]. Figure 6 shows the chemical structures of the aforementioned metabolites.
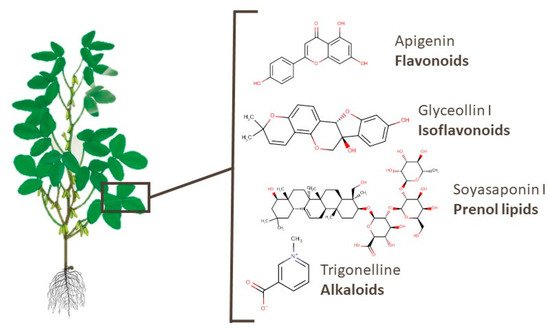
Figure 6. Chemical structures of apigenin, glyceollin I, soyasaponin I, and trigonelline, which are examples of bioactive compounds identified in soy leaves.
4.3. Branches
In soy branches, 197 compounds have already been identified. The most widely reported class among these metabolites is the organooxygen compounds category (53 compounds), such as alcohols and polyols, carbohydrates and their conjugates, and carbonyl. Shikimic acid, an example of an organooxygen compound, was linked to therapeutic effects in osteoarthritis [123]. Metabolites of other classes, such as succinic and stearic acids, presented an apoptotic effect in T-cell acute lymphoblastic leukemia and antifibrotic activity, respectively [124][125]. Flavonoids and isoflavonoids, such as 7,4′-dihydroxyflavone and glycitin, presented activity against lung diseases [126][127]. The chemical structures of these compounds are shown in Figure 7.
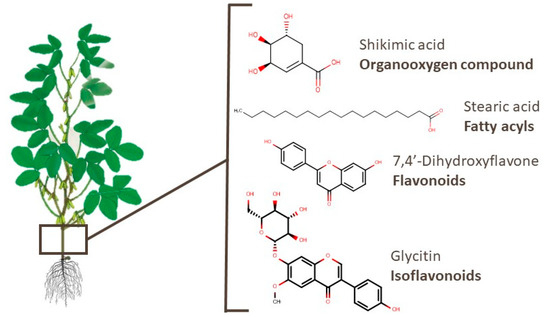
Figure 7. Chemical structures of shikimic acid, stearic acid, 7,4′-dihydroxyflavone, and glycitin, which are examples of bioactive compounds identified in soy branches.
4.4. Pods
Similarly to branches, there are few metabolomics works identifying pod metabolites [38][86][88][92][93][95]. Amino acids, peptides, and mono-, di-, and tricarboxylic acids and their derivatives are the most mentioned types of compounds in pods, with some of these substances already widely used in industry, such as citric and fumaric acids. Moreover, specialized metabolites such as camphene and α-pinene, which were also identified in soy pods, presented anti-skeletal muscle atrophy and neuroprotective effects, respectively [128][129]. Quercetin, which is a widely known flavonoid, may be a potential anti-inflammatory treatment in patients with COVID-19, as described by Saeedi-Boroujeni and Mahmoudian-Sani [130]. Hexadecanoic acid, a fatty acyl compound, presented an inhibitory effect on HT-29 human colon cancer cells [131]. Figure 8 presents the chemical structures of one compound of each class mentioned. In addition, fatty acyls, flavonoids, isoflavonoids, and other classes of compounds were identified in pods.
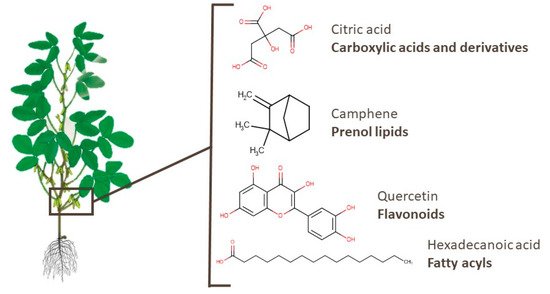
Figure 8. Chemical structures of citric acid, camphene, quercetin, and hexadecanoic acid, which are examples of bioactive compounds identified in soy pods.
References
- Atanasov, A.G.; the International Natural Product Sciences Taskforce; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216.
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353.
- Armenta, S.; Garrigues, S.; Esteve-Turrillas, F.A.; de la Guardia, M. Green extraction techniques in green analytical chemistry. TrAC Trends Anal. Chem. 2019, 116, 248–253.
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized liquid extraction. In Liquid-Phase Extraction; Poole, C.F., Ed.; Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 375–398. ISBN 9780128169117.
- Chai, Y.; Yusup, S.; Kadir, W.; Wong, C.; Rosli, S.; Ruslan, M.; Chin, B.; Yiin, C. Valorization of Tropical Biomass Waste by Supercritical Fluid Extraction Technology. Sustainability 2020, 13, 233.
- Sánchez-Camargo, A.D.P.; Parada-Alonso, F.; Ibáñez, E.; Cifuentes, A. Recent applications of on-line supercritical fluid extraction coupled to advanced analytical techniques for compounds extraction and identification. J. Sep. Sci. 2019, 42, 243–257.
- Keppler, E.A.H.; Jenkins, C.; Davis, T.J.; Bean, H.D. Advances in the application of comprehensive two-dimensional gas chromatography in metabolomics. TrAC Trends Anal. Chem. 2018, 109, 275–286.
- Pirok, B.W.J.; Stoll, D.R.; Schoenmakers, P.J. Recent Developments in Two-Dimensional Liquid Chromatography: Fundamental Improvements for Practical Applications. Anal. Chem. 2019, 91, 240–263.
- Alvarez, G.; Montero, L.; Llorens, L.; Castro-Puyana, M.; Cifuentes, A. Recent advances in the application of capillary electromigration methods for food analysis and Foodomics. Electrophoresis 2018, 39, 136–159.
- Alvarez-Rivera, G.; Ballesteros-Vivas, D.; Parada-Alfonso, F.; Ibañez, E.; Cifuentes, A. Recent applications of high resolution mass spectrometry for the characterization of plant natural products. TrAC Trends Anal. Chem. 2019, 112, 87–101.
- Aksenov, A.A.; Da Silva, R.; Knight, R.; Lopes, N.P.; Dorrestein, P.C. Global chemical analysis of biology by mass spectrometry. Nat. Rev. Chem. 2017, 1, 0054.
- McAlpine, J.B.; Chen, S.-N.; Kutateladze, A.; MacMillan, J.B.; Appendino, G.; Barison, A.; Beniddir, M.A.; Biavatti, M.W.; Bluml, S.; Boufridi, A.; et al. The value of universally available raw NMR data for transparency, reproducibility, and integrity in natural product research. Nat. Prod. Rep. 2019, 36, 35–107.
- Sorokina, M.; Steinbeck, C. Review on natural products databases: Where to find data in 2020. J. Chemin 2020, 12, 1–51.
- Medema, M.H. The year 2020 in natural product bioinformatics: An overview of the latest tools and databases. Nat. Prod. Rep. 2021, 38, 301–306.
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908.
- Reher, R.; Kim, H.W.; Zhang, C.; Mao, H.H.; Wang, M.; Nothias, L.-F.; Caraballo-Rodriguez, A.M.; Glukhov, E.; Teke, B.; Leao, T.; et al. A Convolutional Neural Network-Based Approach for the Rapid Annotation of Molecularly Diverse Natural Products. J. Am. Chem. Soc. 2020, 142, 4114–4120.
- Food and Agriculture Organization of the United Nations FAOSTAT. Available online: (accessed on 2 February 2021).
- Ishangulyyev, R.; Kim, S.; Lee, S.H. Understanding Food Loss and Waste—Why Are We Losing and Wasting Food? Foods 2019, 8, 297.
- Ali, S.S.; Bharadwaj, S. Prospects for Detecting the 326.5 MHz Redshifted 21-Cm HI Signal with the Ooty Radio Telescope (ORT); Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; Volume 35, ISBN 9789251072059.
- UN DP Goal 12: Responsible Consumption and Production; United Nations Development Programme: New York, NY, USA, 2016.
- Cifuentes, A. Food analysis and Foodomics. J. Chromatogr. A 2009, 1216, 7109.
- Valdés, A.; Cifuentes, A.; León, C. Foodomics evaluation of bioactive compounds in foods. TrAC Trends Anal. Chem. 2017, 96, 2–13.
- Katsinas, N.; da Silva, A.B.; Enríquez-De-Salamanca, A.; Fernández, N.; Bronze, M.R.; Rodríguez-Rojo, S. Pressurized Liquid Extraction Optimization from Supercritical Defatted Olive Pomace: A Green and Selective Phenolic Extraction Process. ACS Sustain. Chem. Eng. 2021, 9, 5590–5602.
- Assirati, J.; Rinaldo, D.; Rabelo, S.C.; Bolzani, V.D.S.; Hilder, E.F.; Funari, C.S. A green, simplified, and efficient experimental setup for a high-throughput screening of agri-food by-products—From polar to nonpolar metabolites in sugarcane solid residues. J. Chromatogr. A 2020, 1634, 461693.
- Sánchez-Martínez, J.D.; Bueno, M.; Alvarez-Rivera, G.; Tudela, J.; Ibañez, E.; Cifuentes, A. In vitro neuroprotective potential of terpenes from industrial orange juice by-products. Food Funct. 2021, 12, 302–314.
- Agrawal, M.; Ajazuddin; Tripathi, D.K.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Mourtas, S.; Hammarlund-Udenaes, M.; Alexander, A. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J. Control. Release 2017, 260, 61–77.
- Anderson, E.J.; Ali, M.L.; Beavis, W.D.; Chen, P.; Clemente, T.E.; Diers, B.W.; Graef, G.L.; Grassini, P.; Hyten, D.L.; McHale, L.K.; et al. Soybean [Glycine max (L.) Merr.] Breeding: History, Improvement, Production and Future Opportunities. In Advances in Plant Breeding Strategies: Legumes; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 7, pp. 431–516. ISBN 978-3-030-23400-3.
- United Nations United Nations Statistics Division-Environment Statistics. Available online: (accessed on 20 March 2021).
- United States Department of Agriculture. Oilseeds: World Markets and Trade. 2021. Available online: (accessed on 20 March 2021).
- United States Department of Agriculture. Oilseeds: World Markets and Trade. 2011. Available online: (accessed on 20 March 2021).
- Krisnawati, A.; Adie, M.M. Variability of Biomass and Harvest Index from Several Soybean Genotypes as Renewable Energy Source. Energy Procedia 2015, 65, 14–21.
- Buffett, H.G. Conservation: Reaping the Benefits of No-Tillage Farming. Nature 2012, 484, 455.
- Gawęda, D.; Haliniarz, M.; Bronowicka-Mielniczuk, U.; Łukasz, J. Weed Infestation and Health of the Soybean Crop Depending on Cropping System and Tillage System. Agriculture 2020, 10, 208.
- De Castro, S.G.Q.; Dinardo-Miranda, L.L.; Fracasso, J.V.; Bordonal, R.O.; Menandro, L.; Franco, H.C.J.; Carvalho, J.L.N. Changes in Soil Pest Populations Caused by Sugarcane Straw Removal in Brazil. BioEnergy Res. 2019, 12, 878–887.
- Zhang, J.; Hang, X.; Lamine, S.M.; Jiang, Y.; Afreh, D.; Qian, H.; Feng, X.; Zheng, C.; Deng, A.; Song, Z.; et al. Interactive effects of straw incorporation and tillage on crop yield and greenhouse gas emissions in double rice cropping system. Agric. Ecosyst. Environ. 2017, 250, 37–43.
- Popin, G.V.; Santos, A.K.B.; Oliveira, T.D.P.; De Camargo, P.B.; Cerri, C.E.; Siqueira-Neto, M. Sugarcane straw management for bioenergy: Effects of global warming on greenhouse gas emissions and soil carbon storage. Mitig. Adapt. Strat. Glob. Chang. 2020, 25, 559–577.
- Vasconcelos, A.L.S.; Cherubin, M.R.; Feigl, B.J.; Cerri, C.E.; Gmach, M.R.; Siqueira-Neto, M. Greenhouse gas emission responses to sugarcane straw removal. Biomass Bioenergy 2018, 113, 15–21.
- Carneiro, A.M.; Moreira, E.A.; Bragagnolo, F.S.; Borges, M.S.; Pilon, A.C.; Rinaldo, D.; De Funari, C.S. Soya agricultural waste as a rich source of isoflavones. Food Res. Int. 2020, 130, 108949.
- Lu, Y.; Lam, H.-M.; Pi, E.; Zhan, Q.; Tsai, S.; Wang, C.; Kwan, Y.; Ngai, S. Comparative Metabolomics in Glycine Max and Glycine soja under Salt Stress To Reveal the Phenotypes of Their Offspring. J. Agric. Food Chem. 2013, 61, 8711–8721.
- Yun, D.-Y.; Kang, Y.-G.; Yun, B.; Kim, E.-H.; Kim, M.; Park, J.S.; Lee, J.H.; Hong, Y.-S. Distinctive Metabolism of Flavonoid between Cultivated and Semiwild Soybean Unveiled through Metabolomics Approach. J. Agric. Food Chem. 2016, 64, 5773–5783.
- Patent Database Search Results: TTL/“Soybean Cultivar” OR TTL/“Soybean Variety” in US Patent Collection. Available online: (accessed on 10 May 2021).
- Lee, J.; Hwang, Y.-S.; Kim, S.T.; Yoon, W.-B.; Han, W.Y.; Kang, I.-K.; Choung, M.-G. Seed coat color and seed weight contribute differential responses of targeted metabolites in soybean seeds. Food Chem. 2017, 214, 248–258.
- Gupta, R.; Min, C.W.; Kim, S.W.; Wang, Y.; Agrawal, G.K.; Rakwal, R.; Kim, S.G.; Lee, B.W.; Ko, J.M.; Baek, I.Y.; et al. Comparative investigation of seed coats of brown- versus yellow-colored soybean seeds using an integrated proteomics and metabolomics approach. Proteomics 2015, 15, 1706–1716.
- Yang, C.-Q.; Zheng, L.; Wu, H.-J.; Zhu, Z.-K.; Zou, Y.-F.; Deng, J.-C.; Qin, W.-T.; Zhang, J.; Wang, X.-C.; Yang, W.-Y.; et al. Yellow- and green-cotyledon seeds of black soybean: Phytochemical and bioactive differences determine edibility and medical applications. Food Biosci. 2021, 39, 100842.
- Yun, Y.J.; Lee, H.; Yoo, D.J.; Yang, J.Y.; Woo, S.-Y.; Seo, W.D.; Kim, Y.-C.; Lee, J.H. Molecular analysis of soyasaponin biosynthetic genes in two soybean (Glycine Max L. Merr.) cultivars. Plant Biotechnol. Rep. 2021, 15, 117–124.
- García-Villalba, R.; León, C.; Dinelli, G.; Carretero, A.S.; Fernández-Gutiérrez, A.; Garcia-Cañas, V.; Cifuentes, A. Comparative metabolomic study of transgenic versus conventional soybean using capillary electrophoresis–time-of-flight mass spectrometry. J. Chromatogr. A 2008, 1195, 164–173.
- Harrigan, G.G.; Skogerson, K.; MacIsaac, S.; Bickel, A.; Perez, T.; Li, X. Application of 1H NMR Profiling To Assess Seed Metabolomic Diversity. A Case Study on a Soybean Era Population. J. Agric. Food Chem. 2015, 63, 4690–4697.
- Clarke, J.D.; Alexander, D.C.; Ward, D.P.; Ryals, J.A.; Mitchell, M.W.; Wulff, J.E.; Guo, L. Assessment of Genetically Modified Soybean in Relation to Natural Variation in the Soybean Seed Metabolome. Sci. Rep. 2013, 3, 3082.
- de Campos, B.K.; Galazzi, R.M.; dos Santos, B.M.; Balbuena, T.S.; dos Santos, F.N.; Mokochinski, J.B.; Eberlin, M.N.; Arruda, M.A. Comparison of generational effect on proteins and metabolites in non-transgenic and transgenic soybean seeds through the insertion of the cp4-EPSPS gene assessed by omics-based platforms. Ecotoxicol. Environ. Saf. 2020, 202, 110918.
- Rodrigues, J.M.; Coutinho, F.S.; dos Santos, D.S.; Vital, C.E.; Ramos, J.R.L.S.; Reis, P.B.; Oliveira, M.G.A.; Mehta, A.; Fontes, E.P.B.; Ramos, H.J.O. BiP-overexpressing soybean plants display accelerated hypersensitivity response (HR) affecting the SA-dependent sphingolipid and flavonoid pathways. Phytochemistry 2021, 185, 112704.
- Kang, W.-S.; Chen, L.-J.; Wang, Y.-Y.; Zhu, X.-F.; Liu, X.-Y.; Fan, H.-Y.; Duan, Y.-X. Bacillus simplex treatment promotes soybean defence against soybean cyst nematodes: A metabolomics study using GC-MS. PLoS ONE 2020, 15, e0237194.
- Nakata, R.; Yano, M.; Hiraga, S.; Teraishi, M.; Okumoto, Y.; Mori, N.; Kaga, A. Molecular Basis Underlying Common Cutworm Resistance of the Primitive Soybean Landrace Peking. Front. Genet. 2020, 11, 11.
- Zhang, P.; Du, H.; Wang, J.; Pu, Y.; Yang, C.; Yan, R.; Yang, H.; Cheng, H.; Yu, D. Multiplex CRISPR/Cas9-mediated metabolic engineering increases soya bean isoflavone content and resistance to soya bean mosaic virus. Plant Biotechnol. J. 2019, 18, 1384–1395.
- Ding, X.; Wang, X.; Li, Q.; Yu, L.; Song, Q.; Gai, J.; Yang, S. Metabolomics Studies on Cytoplasmic Male Sterility during Flower Bud Development in Soybean. Int. J. Mol. Sci. 2019, 20, 2869.
- Ranjan, A.; Westrick, N.M.; Jain, S.; Piotrowski, J.S.; Ranjan, M.; Kessens, R.; Stiegman, L.; Grau, C.R.; Conley, S.; Smith, D.L.; et al. Resistance against Sclerotinia sclerotiorum in soybean involves a reprogramming of the phenylpropanoid pathway and up-regulation of antifungal activity targeting ergosterol biosynthesis. Plant Biotechnol. J. 2019, 17, 1567–1581.
- de Oliveira, C.S.; Lião, L.M.; Alcantara, G.B. Metabolic response of soybean plants to Sclerotinia sclerotiorum infection. Phytochemistry 2019, 167, 112099.
- Cui, J.-Q.; Sun, H.-B.; Sun, M.-B.; Liang, R.-T.; Jie, W.-G.; Cai, B.-Y. Effects of Funneliformis mosseae on Root Metabolites and Rhizosphere Soil Properties to Continuously-Cropped Soybean in the Potted-Experiments. Int. J. Mol. Sci. 2018, 19, 2160.
- Zhu, L.; Zhou, Y.; Li, X.; Zhao, J.; Guo, N.; Xing, H. Metabolomics Analysis of Soybean Hypocotyls in Response to Phytophthora sojae Infection. Front. Plant Sci. 2018, 9, 1530.
- Kang, W.; Zhu, X.; Wang, Y.; Chen, L.; Duan, Y. Transcriptomic and metabolomic analyses reveal that bacteria promote plant defense during infection of soybean cyst nematode in soybean. BMC Plant Biol. 2018, 18, 1–14.
- Copley, T.R.; Aliferis, K.A.; Kliebenstein, D.J.; Jabaji, S.H. An integrated RNAseq-1H NMR metabolomics approach to understand soybean primary metabolism regulation in response to Rhizoctonia foliar blight disease. BMC Plant Biol. 2017, 17, 1–18.
- Lardi, M.; Murset, V.; Fischer, H.-M.; Mesa, S.; Ahrens, C.H.; Zamboni, N.; Pessi, G. Metabolomic Profiling of Bradyrhizobium diazoefficiens-Induced Root Nodules Reveals Both Host Plant-Specific and Developmental Signatures. Int. J. Mol. Sci. 2016, 17, 815.
- Abeysekara, N.S.; Swaminathan, S.; Desai, N.; Guo, L.; Bhattacharyya, M.K. The plant immunity inducer pipecolic acid accumulates in the xylem sap and leaves of soybean seedlings following Fusarium virguliforme infection. Plant Sci. 2016, 243, 105–114.
- Scandiani, M.M.; Luque, A.G.; Razori, M.V.; Casalini, L.C.; Aoki, T.; O’Donnell, K.; Cervigni, G.D.L.; Spampinato, C.P. Metabolic profiles of soybean roots during early stages of Fusarium tucumaniae infection. J. Exp. Bot. 2014, 66, 391–402.
- Sato, D.; Akashi, H.; Sugimoto, M.; Tomita, M.; Soga, T. Metabolomic profiling of the response of susceptible and resistant soybean strains to foxglove aphid, Aulacorthum solani Kaltenbach. J. Chromatogr. B 2013, 925, 95–103.
- Brechenmacher, L.; Lei, Z.; Libault, M.; Findley, S.; Sugawara, M.; Sadowsky, M.J.; Sumner, L.W.; Stacey, G. Soybean Metabolites Regulated in Root Hairs in Response to the Symbiotic Bacterium Bradyrhizobium japonicum. Plant Physiol. 2010, 153, 1808–1822.
- Silva, E.; Perez Da Graça, J.; Porto, C.; Martin Do Prado, R.; Nunes, E.; Corrêa Marcelino-Guimarães, F.; Conrado Meyer, M.; Jorge Pilau, E. Untargeted Metabolomics Analysis by UHPLC-MS/MS of Soybean Plant in a Compatible Response to Phakopsora Pachyrhizi Infection. Metabolites 2021, 11, 179.
- Zanzarin, D.M.; Hernandes, C.P.; Leme, L.M.; Silva, E.; Porto, C.; Prado, R.M.D.; Meyer, M.C.; Favoreto, L.; Nunes, E.; Pilau, E.J. Metabolomics of soybean green stem and foliar retention (GSFR) disease using mass spectrometry and molecular networking. Rapid Commun. Mass Spectrom. 2019, 34, e8655.
- Silva, E.; Da Graça, J.P.; Porto, C.; Prado, R.M.D.; Hoffmann-Campo, C.B.; Meyer, M.C.; Nunes, E.; Pilau, E.J. Unraveling Asian Soybean Rust metabolomics using mass spectrometry and Molecular Networking approach. Sci. Rep. 2020, 10, 138.
- Lee, J.; Hwang, Y.S.; Chang, W.S.; Moon, J.K.; Choung, M.G. Seed Maturity Differentially Mediates Metabolic Responses in Black Soybean. Food Chem. 2013, 141, 2052–2059.
- Collakova, E.; Aghamirzaie, D.; Fang, Y.; Klumas, C.; Tabataba, F.; Kakumanu, A.; Myers, E.; Heath, L.S.; Grene, R. Metabolic and Transcriptional Reprogramming in Developing Soybean (Glycine Max) Embryos. Metabolites 2013, 3, 347–372.
- Li, L.; Hur, M.; Lee, J.Y.; Zhou, W.; Song, Z.; Ransom, N.; Demirkale, C.Y.; Nettleton, D.; Westgate, M.; Arendsee, Z.; et al. A Systems Biology Approach toward Understanding Seed Composition in Soybean. BMC Genom. 2015, 16, S9.
- Gu, E.J.; Kim, D.W.; Jang, G.J.; Song, S.H.; Lee, J.I.; Lee, S.B.; Kim, B.M.; Cho, Y.; Lee, H.J.; Kim, H.J. Mass-Based Metabolomic Analysis of Soybean Sprouts during Germination. Food Chem. 2017, 217, 311–319.
- Song, H.H.; Ryu, H.W.; Lee, K.J.; Jeong, I.Y.; Kim, D.S.; Oh, S.R. Metabolomics Investigation of Flavonoid Synthesis in Soybean Leaves Depending on the Growth Stage. Metabolomics 2014, 10, 833–841.
- Makino, Y.; Nishizaka, A.; Yoshimura, M.; Sotome, I.; Kawai, K.; Akihiro, T. Influence of Low O2 and High CO2 Environment on Changes in Metabolite Concentrations in Harvested Vegetable Soybeans. Food Chem. 2020, 317, 126380.
- Pi, E.; Zhu, C.; Fan, W.; Huang, Y.; Qu, L.; Li, Y.; Zhao, Q.; Ding, F.; Qiu, L.; Wang, H.; et al. Quantitative Phosphoproteomic and Metabolomic Analyses Reveal GmMYB173 Optimizes Flavonoid Metabolism in Soybean under Salt Stress. Mol. Cell. Proteom. 2018, 17, 1209–1224.
- Das, A.; Rushton, P.J.; Rohila, J.S. Metabolomic Profiling of Soybeans (Glycine Max L.) Reveals the Importance of Sugar and Nitrogen Metabolism under Drought and Heat Stress. Plants 2017, 6, 21.
- Zou, J.; Yu, H.; Yu, Q.; Jin, X.; Cao, L.; Wang, M.; Wang, M.; Ren, C.; Zhang, Y. Physiological and UPLC-MS/MS Widely Targeted Metabolites Mechanisms of Alleviation of Drought Stress-Induced Soybean Growth Inhibition by Melatonin. Ind. Crop. Prod. 2021, 163, 113323.
- Gupta, R.; Min, C.W.; Kramer, K.; Agrawal, G.K.; Rakwal, R.; Park, K.H.; Wang, Y.; Finkemeier, I.; Kim, S.T. A Multi-Omics Analysis of Glycine Max Leaves Reveals Alteration in Flavonoid and Isoflavonoid Metabolism Upon Ethylene and Abscisic Acid Treatment. Proteomics 2018, 18, 1700366.
- Cheng, J.; Yuan, C.; Graham, T.L. Potential Defense-Related Prenylated Isoflavones in Lactofen-Induced Soybean. Phytochemistry 2011, 72, 875–881.
- Li, Y.; Zhang, Q.; Yu, Y.; Li, X.; Tan, H. Integrated proteomics, metabolomics and physiological analyses for dissecting the toxic effects of halosulfuron-methyl on soybean seedlings (Glycine Max merr.). Plant Physiol. Biochem. 2020, 157, 303–315.
- Zhong, Z.; Kobayashi, T.; Zhu, W.; Imai, H.; Zhao, R.; Ohno, T.; Rehman, S.U.; Uemura, M.; Tian, J.; Komatsu, S. Plant-derived smoke enhances plant growth through ornithine-synthesis pathway and ubiquitin-proteasome pathway in soybean. J. Proteom. 2020, 221, 103781.
- Ban, Y.J.; Song, Y.H.; Kim, J.Y.; Baiseitova, A.; Lee, K.W.; Kim, K.D.; Park, K.H. Comparative investigation on metabolites changes in soybean leaves by ethylene and activation of collagen synthesis. Ind. Crop. Prod. 2020, 154, 112743.
- Yilmaz, A.; Rudolph, H.L.; Hurst, J.J.; Wood, T.D. High-Throughput Metabolic Profiling of Soybean Leaves by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Anal. Chem. 2015, 88, 1188–1194.
- Yun, D.-Y.; Kang, Y.-G.; Kim, E.-H.; Kim, M.; Park, N.-H.; Choi, H.-T.; Go, G.H.; Lee, J.H.; Park, J.S.; Hong, Y.-S. Metabolomics approach for understanding geographical dependence of soybean leaf metabolome. Food Res. Int. 2018, 106, 842–852.
- Feng, Z.; Ding, C.; Li, W.; Wang, D.; Cui, D. Applications of metabolomics in the research of soybean plant under abiotic stress. Food Chem. 2020, 310, 125914.
- Seo, W.D.; Kang, J.E.; Choi, S.-W.; Lee, K.-S.; Lee, M.-J.; Park, K.-D.; Lee, J.H. Comparison of nutritional components (isoflavone, protein, oil, and fatty acid) and antioxidant properties at the growth stage of different parts of soybean [Glycine Max (L.) Merrill]. Food Sci. Biotechnol. 2017, 26, 339–347.
- Jiang, Z.-F.; Liu, D.-D.; Wang, T.-Q.; Liang, X.-L.; Cui, Y.-H.; Liu, Z.-H.; Li, W.-B. Concentration difference of auxin involved in stem development in soybean. J. Integr. Agric. 2020, 19, 953–964.
- Hu, B.-Y.; Yang, C.-Q.; Iqbal, N.; Deng, J.-C.; Zhang, J.; Yang, W.-Y.; Liu, J. Development and validation of a GC–MS method for soybean organ-specific metabolomics. Plant Prod. Sci. 2018, 21, 215–224.
- Dresler, S.; Wójciak-Kosior, M.; Sowa, I.; Strzemski, M.; Sawicki, J.; Kováčik, J.; Blicharski, T. Effect of Long-Term Strontium Exposure on the Content of Phytoestrogens and Allantoin in Soybean. Int. J. Mol. Sci. 2018, 19, 3864.
- Nam, K.-H.; Kim, D.Y.; Kim, H.J.; Pack, I.-S.; Chung, Y.S.; Kim, S.Y.; Kim, C.-G. Global metabolite profiling based on GC–MS and LC–MS/MS analyses in ABF3-overexpressing soybean with enhanced drought tolerance. Appl. Biol. Chem. 2019, 62, 15.
- Coutinho, I.D.; Henning, L.M.M.; Döpp, S.A.; Nepomuceno, A.; Moraes, L.A.C.; Marcolino-Gomes, J.; Richter, C.; Schwalbe, H.; Colnago, L.A. Identification of primary and secondary metabolites and transcriptome profile of soybean tissues during different stages of hypoxia. Data Brief 2018, 21, 1089–1100.
- Silva, F.A.C.; Carrão-Panizzi, M.C.; Blassioli-Moraes, M.C.; Panizzi, A.R. Influence of Volatile and Nonvolatile Secondary Metabolites From Soybean Pods on Feeding and on Oviposition Behavior of Euschistus heros (Hemiptera: Heteroptera: Pentatomidae). Environ. Entomol. 2013, 42, 1375–1382.
- Deng, J.-C.; Yang, C.-Q.; Zhang, J.; Zhang, Q.; Yang, F.; Yang, W.-Y.; Liu, J. Organ-Specific Differential NMR-Based Metabonomic Analysis of Soybean [Glycine Max (L.) Merr.] Fruit Reveals the Metabolic Shifts and Potential Protection Mechanisms Involved in Field Mold Infection. Front. Plant Sci. 2017, 8.
- Li, M.; Xu, J.; Wang, X.; Fu, H.; Zhao, M.; Wang, H.; Shi, L. Photosynthetic characteristics and metabolic analyses of two soybean genotypes revealed adaptive strategies to low-nitrogen stress. J. Plant Physiol. 2018, 229, 132–141.
- Dillon, F.M.; Chludil, H.D.; Mithöfer, A.; Zavala, J.A. Solar UVB-inducible ethylene alone induced isoflavonoids in pods of field-grown soybean, an important defense against stink bugs. Environ. Exp. Bot. 2020, 178, 104167.
- Tsuno, Y.; Fujimatsu, T.; Endo, K.; Sugiyama, A.; Yazaki, K. Soyasaponins: A New Class of Root Exudates in Soybean (Glycine Max). Plant Cell Physiol. 2018, 59, 366–375.
- ChemAxon-Software Solutions and Services for Chemistry & Biology. Available online: (accessed on 8 April 2021).
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated Chemical Classification with a Comprehensive, Computable Taxonomy. J. Chemin. 2016, 8, 1–20.
- Salama, A.A.A.; Allam, R.M. Promising Targets of Chrysin and Daidzein in Colorectal Cancer: Amphiregulin, CXCL1, and MMP-9. Eur. J. Pharmacol. 2021, 892, 173763.
- He, Y.; Huang, M.; Tang, C.; Yue, Y.; Liu, X.; Zheng, Z.; Dong, H.; Liu, D. Dietary Daidzein Inhibits Hepatitis C Virus Replication by Decreasing MicroRNA-122 Levels. Virus Res. 2021, 298, 198404.
- Yang, M.H.; Jung, S.H.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Sethi, G.; Ahn, K.S. Attenuation of STAT3 Signaling Cascade by Daidzin Can Enhance the Apoptotic Potential of Bortezomib against Multiple Myeloma. Biomolecules 2020, 10, 23.
- Kazmi, Z.; Zeeshan, S.; Khan, A.; Malik, S.; Shehzad, A.; Seo, E.K.; Khan, S. Anti-Epileptic Activity of Daidzin in PTZ-Induced Mice Model by Targeting Oxidative Stress and BDNF/VEGF Signaling. NeuroToxicology 2020, 79, 150–163.
- Xie, X.; Cong, L.; Liu, S.; Xiang, L.; Fu, X. Genistein Alleviates Chronic Vascular Inflammatory Response via the MiR-21/NF-ΚB P65 Axis in Lipopolysaccharide-Treated Mice. Mol. Med. Rep. 2021, 23, 192.
- Zang, Y.Q.; Feng, Y.Y.; Luo, Y.H.; Zhai, Y.Q.; Ju, X.Y.; Feng, Y.C.; Wang, J.R.; Yu, C.Q.; Jin, C.H. Glycitein Induces Reactive Oxygen Species-Dependent Apoptosis and G0/G1 Cell Cycle Arrest through the MAPK/STAT3/NF-ΚB Pathway in Human Gastric Cancer Cells. Drug Dev. Res. 2019, 80, 573–584.
- Hwang, S.T.; Yang, M.H.; Baek, S.H.; Um, J.Y.; Ahn, K.S. Genistin Attenuates Cellular Growth and Promotes Apoptotic Cell Death Breast Cancer Cells through Modulation of ERalpha Signaling Pathway. Life Sci. 2020, 263, 118594.
- Zhou, Y.; Xu, B.; Yu, H.; Zhao, W.; Song, X.; Liu, Y.; Wang, K.; Peacher, N.; Zhao, X.; Zhang, H.T. Biochanin A Attenuates Ovariectomy-Induced Cognition Deficit via Antioxidant Effects in Female Rats. Front. Pharmacol. 2021, 12, 171.
- Xu, Y.; Zhang, Y.; Liang, H.; Liu, X. Coumestrol Mitigates Retinal Cell Inflammation, Apoptosis, and Oxidative Stress in a Rat Model of Diabetic Retinopathy via Activation of SIRT1. Aging 2021, 13, 5342–5357.
- Yamamoto, T.; Sakamoto, C.; Tachiwana, H.; Kumabe, M.; Matsui, T.; Yamashita, T.; Shinagawa, M.; Ochiai, K.; Saitoh, N.; Nakao, M. Endocrine Therapy-Resistant Breast Cancer Model Cells Are Inhibited by Soybean Glyceollin I through Eleanor Non-Coding RNA. Sci. Rep. 2018, 8, 1–12.
- Mansoori, M.N.; Raghuvanshi, A.; Shukla, P.; Awasthi, P.; Trivedi, R.; Goel, A.; Singh, D. Medicarpin Prevents Arthritis in Post-Menopausal Conditions by Arresting the Expansion of TH17 Cells and pro-Inflammatory Cytokines. Int. Immunopharmacol. 2020, 82, 106299.
- Luo, L.; Zhou, J.; Zhao, H.; Fan, M.; Gao, W. The Anti-Inflammatory Effects of Formononetin and Ononin on Lipopolysaccharide-Induced Zebrafish Models Based on Lipidomics and Targeted Transcriptomics. Metabolomics 2019, 15, 153.
- Tsai, W.; Nakamura, Y.; Akasaka, T.; Katakura, Y.; Tanaka, Y.; Shirouchi, B.; Jiang, Z.; Yuan, X.; Sato, M. Soyasaponin Ameliorates Obesity and Reduces Hepatic Triacylglycerol Accumulation by Suppressing Lipogenesis in High-fat Diet-fed Mice. J. Food Sci. 2021.
- Kim, H.J.; Choi, E.J.; Kim, H.S.; Choi, C.W.; Choi, S.W.; Kim, S.L.; Seo, W.D.; Do, S.H. Soyasaponin Ab Alleviates Postmenopausal Obesity through Browning of White Adipose Tissue. J. Funct. Foods 2019, 57, 453–464.
- Liu, X.; Chen, K.; Zhu, L.; Liu, H.; Ma, T.; Xu, Q.; Xie, T. Soyasaponin Ab Protects against Oxidative Stress in HepG2 Cells via Nrf2/HO-1/NQO1 Signaling Pathways. J. Funct. Foods 2018, 45, 110–117.
- Wang, F.; Gong, S.; Wang, T.; Li, L.; Luo, H.; Wang, J.; Huang, C.; Zhou, H.; Chen, G.; Liu, Z.; et al. Soyasaponin II Protects against Acute Liver Failure through Diminishing YB-1 Phosphorylation and Nlrp3-Inflammasome Priming in Mice. Theranostics 2020, 10, 2714–2726.
- Omar, A.; Kalra, R.S.; Putri, J.; Elwakeel, A.; Kaul, S.C.; Wadhwa, R. Soyasapogenol-A Targets CARF and Results in Suppression of Tumor Growth and Metastasis in P53 Compromised Cancer Cells. Sci. Rep. 2020, 10, 1–13.
- Jandacek, R.J. Linoleic Acid: A Nutritional Quandary. Healthcare 2017, 5, 25.
- Nouri, Z.; Fakhri, S.; El-Senduny, F.F.; Sanadgol, N.; Abd-Elghani, G.E.; Farzaei, M.H.; Chen, J.T. On the Neuroprotective Effects of Naringenin: Pharmacological Targets, Signaling Pathways, Molecular Mechanisms, and Clinical Perspective. Biomolecules 2019, 9, 690.
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an Anticancer Agent. Phytother. Res. 2020, 34, 1812–1828.
- Guo, Z.; Cao, W.; Zhao, S.; Han, Z.; Han, B. Protection against 1-Methyl-4-Phenyl Pyridinium-Induced Neurotoxicity in Human Neuroblastoma SH-SY5Y Cells by Soyasaponin i by the Activation of the Phosphoinositide 3-Kinase/AKT/GSK3β Pathway. NeuroReport 2016, 27, 730–736.
- Fouzder, C.; Mukhuty, A.; Mukherjee, S.; Malick, C.; Kundu, R. Trigonelline Inhibits Nrf2 via EGFR Signalling Pathway and Augments Efficacy of Cisplatin and Etoposide in NSCLC Cells. Toxicol. Vitr. 2021, 70, 105038.
- Choi, M.; Mukherjee, S.; Yun, J.W. Trigonelline Induces Browning in 3T3-L1 White Adipocytes. Phytother. Res. 2021, 35, 1113–1124.
- Farid, M.M.; Yang, X.; Kuboyama, T.; Tohda, C. Trigonelline Recovers Memory Function in Alzheimer’s Disease Model Mice: Evidence of Brain Penetration and Target Molecule. Sci. Rep. 2020, 10, 16424.
- Guo, X.; Wang, L.; Xu, M.; Bai, J.; Shen, J.; Yu, B.; Liu, Y.; Sun, H.; Hao, Y.; Geng, D. Shikimic Acid Prevents Cartilage Matrix Destruction in Human Chondrocytes. Int. Immunopharmacol. 2018, 63, 155–160.
- Ertugrul, B.; Iplik, E.S.; Cakmakoglu, B. In Vitro Inhibitory Effect of Succinic Acid on T-Cell Acute Lymphoblastic Leukemia Cell Lines. Arch. Med. Res. 2021, 52, 270–276.
- Kim, H.S.; Yoo, H.J.; Lee, K.M.; Song, H.E.; Kim, S.J.; Lee, J.O.; Hwang, J.J.; Song, J.W. Stearic Acid Attenuates Profibrotic Signalling in Idiopathic Pulmonary Fibrosis. Respirology 2021, 26, 255–263.
- Liu, C.; Weir, D.; Busse, P.; Yang, N.; Zhou, Z.; Emala, C.; Li, X.M. The Flavonoid 7,4′-Dihydroxyflavone Inhibits MUC5AC Gene Expression, Production, and Secretion via Regulation of NF-ΚB, STAT6, and HDAC2. Phytother. Res. 2015, 29, 925–932.
- Chen, Y.; Guo, S.; Jiang, K.; Wang, Y.; Yang, M.; Guo, M. Glycitin Alleviates Lipopolysaccharide-Induced Acute Lung Injury via Inhibiting NF-ΚB and MAPKs Pathway Activation in Mice. Int. Immunopharmacol. 2019, 75, 105749.
- Baek, S.; Kim, J.; Moon, B.S.; Park, S.M.; Jung, D.E.; Kang, S.Y.; Lee, S.J.; Oh, S.J.; Kwon, S.H.; Nam, M.H.; et al. Camphene Attenuates Skeletal Muscle Atrophy by Regulating Oxidative Stress and Lipid Metabolism in Rats. Nutrients 2020, 12, 3731.
- Khoshnazar, M.; Parvardeh, S.; Bigdeli, M.R. Alpha-Pinene Exerts Neuroprotective Effects via Anti-Inflammatory and Anti-Apoptotic Mechanisms in a Rat Model of Focal Cerebral Ischemia-Reperfusion. J. Stroke Cerebrovasc. Dis. 2020, 29, 104977.
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R. Anti-Inflammatory Potential of Quercetin in COVID-19 Treatment. J. Inflamm. 2021, 18, 1–9.
- Bharath, B.; Perinbam, K.; Devanesan, S.; AlSalhi, M.S.; Saravanan, M. Evaluation of the Anticancer Potential of Hexadecanoic Acid from Brown Algae Turbinaria Ornata on HT–29 Colon Cancer Cells. J. Mol. Struct. 2021, 1235, 130229.




