Soy has been recognized as a medicinal plant since it contains several bioactive compounds in its various parts. For example, bioactive peptides found in soybeans have been linked to human health benefits with potential anti-hypertensive, anti-cancer, and anti-inflammatory properties. Another type of bioactive compound identified in soybeans, the anthocyanins, showed anti-obesity and anti- inflammatory properties. Isoflavonoids, the best-known class of compounds found in all parts of soy, have been studied due to their potential protective effects associated with chronic diseases, cancer, osteoporosis, and menopausal symptoms. Different factors modulate a plant’s metabolism, and metabolomics can measure these variations qualitatively and quantitatively, analyzing the production and turnover of primary and secondary (specialized) metabolites. In soy, metabolomics studies have identified four main causes of changes in metabolism: genetic modifications, organism interactions, growth stages, and abiotic factors.
- Glycine max
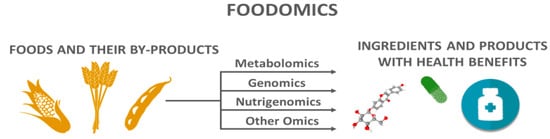
- foodomics
- Soy
1. Metabolomics Applied to Agri-Foods and Their By-Products
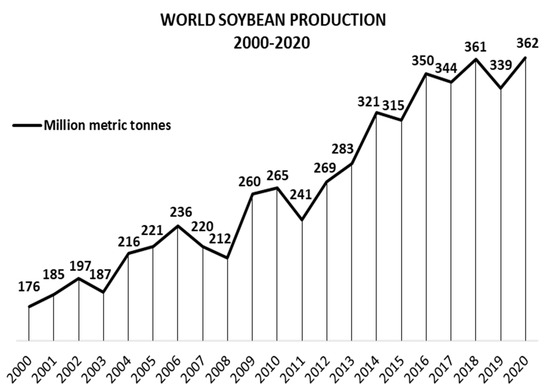
2. Glycine max: More Than Beans
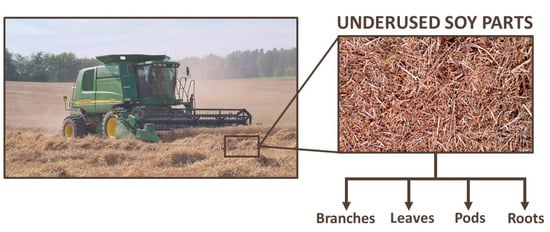
3. Metabolomics and Soy
4. Bioactive Compounds in Underused Soy Parts
| Name | Formula | B | L | P | R | References |
|---|---|---|---|---|---|---|
| 2′-hydroxydaidzein | C15H10O5 | X | [80] | |||
| 7,3′,4′-trihydroxyisoflavone | C15H10O5 | X | [79] | |||
| 7-O-methylluteone | C21H20O6 | X | [78] | |||
| acetyl daidzin | C22H22O9 | X | [104] | |||
| acetyl genistin | C23H22O11 | X | X | [94,104] | ||
| acetyl glycitin | C24H24O11 | X | [104] | |||
| afrormosin 7-O-glucoside | C23H24O10 | X | [80] | |||
| biochanin A | C16H12O5 | X | [80] | |||
| biochanin A 7-O-D-glucoside | C22H22O10 | X | [80] | |||
| biochanin A 7-O-glucoside-6′′-O-malonate | C25H24O13 | X | [80] | |||
| calycosin | C16H12O5 | X | [80] | |||
| coumestrol | C15H8O5 | X | X | [79,80,101] | ||
| daidzein | C15H10O4 | X | X | X | X | [38,78,79,80,94,98,101,103,104,106,107,108] |
| daidzin | C21H20O9 | X | X | X | X | [38,78,79,80,94,98,101,103,104,107,108] |
| formononetin | C16H12O4 | X | [80,102] | |||
| formononetin 7-O-glucoside | C22H22O9 | X | X | [79,80] | ||
| formononetin 7-O-glucoside-6′′-malonate | C25H24O12 | X | [78,80,94] | |||
| formononetin 7-O-glucoside-6-O-malonate | C25H24O12 | X | X | [78,79] | ||
| genistein | C15H10O5 | X | X | X | X | [38,79,94,98,104,108] |
| genistin | C21H20O10 | X | X | X | X | [38,78,79,94,101,104,107,108] |
| glyceollidin I/II | C20H20O5 | X | [80] | |||
| glyceollin I | C20H18O5 | X | [78,80] | |||
| glyceollin II | C20H18O5 | X | [78,80] | |||
| glyceollin III | C20H18O5 | X | [78,80] | |||
| glyceollin IV | C21H22O5 | X | [80] | |||
| glyceollin VI | C20H16O4 | X | [80] | |||
| glycitein | C16H12O5 | X | X | X | X | [38,80,98,104,108] |
| glycitein 7-O-glucoside | C22H22O10 | X | [80] | |||
| glycitin | C22H22O10 | X | X | X | X | [38,79,101,104,108] |
| isotrifoliol | C16H10O6 | X | [80] | |||
| malonyldaidzin | C24H22O12 | X | X | X | X | [38,78,79,80,94,101,103,104,107,108] |
| malonylgenistin | C24H22O13 | X | X | X | X | [78,79,80,94,101,104,107,108] |
| malonylglycitin | C25H24O13 | X | X | X | [80,94,104,108] | |
| medicarpin | C16H14O4 | X | [80] | |||
| neobavaisoflavone | C20H18O4 | X | X | [78,79] | ||
| phaseollin | C20H18O4 | X | [80] | |||
| pisatin | C17H14O6 | X | [80] | |||
| sojagol | C20H16O5 | X | [78,80] |
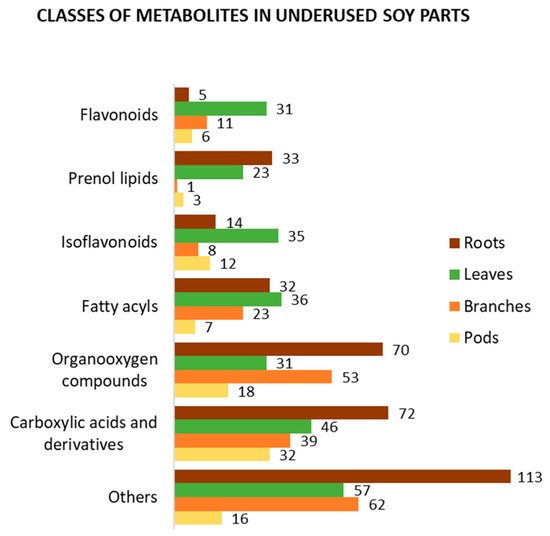
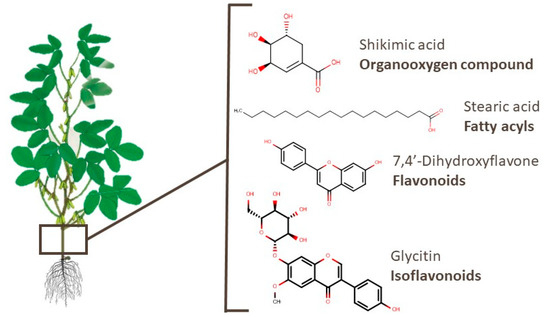
4.1. Roots
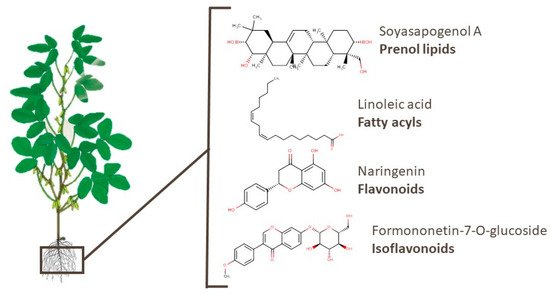
4.2. Leaves
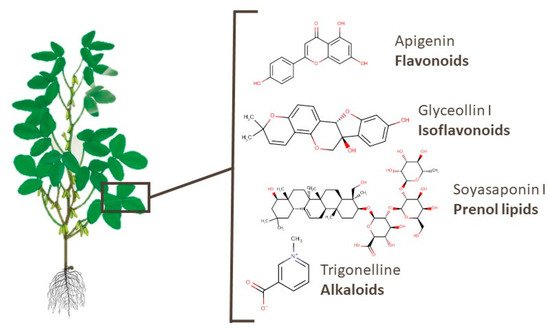
4.3. Branches
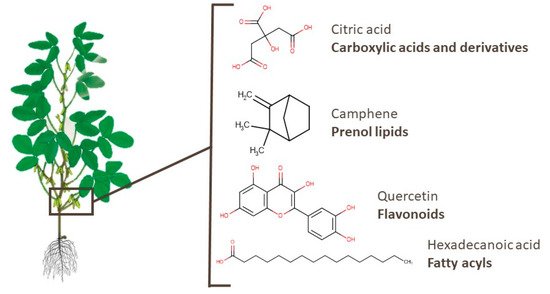
4.4. Pods
This entry is adapted from the peer-reviewed paper 10.3390/foods10061308
